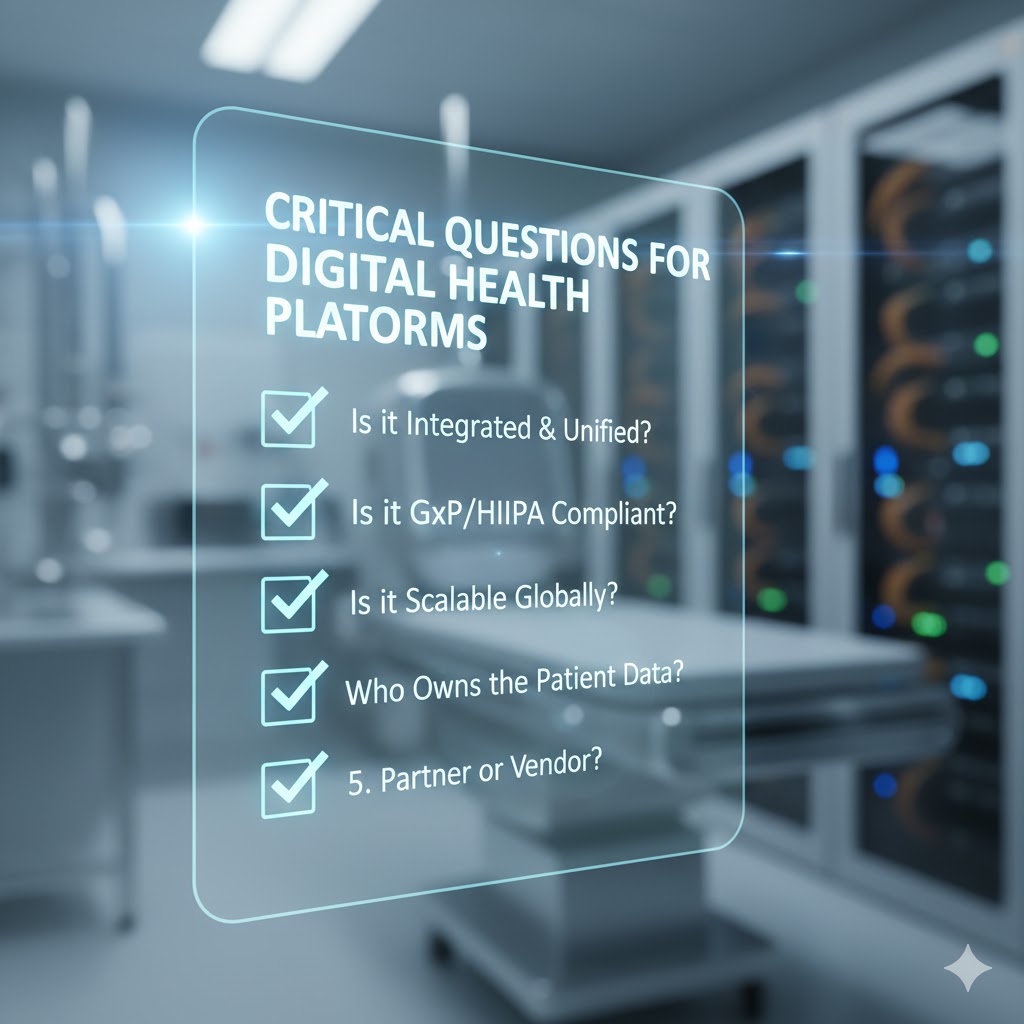
Time-Saving Strategies for Faster, More Efficient Clinical Trials
Running a clinical trial is a complex and highly regulated process—but it doesn’t have to be slow. From protocol development to database lock, there are opportunities to streamline every phase of a study without compromising on quality or compliance.
In this guide, we’ll explore actionable strategies and tools sponsors and CROs can use to accelerate their trials, reduce bottlenecks, and bring therapies to market faster.
Why Speed Matters in Clinical Research
Accelerating clinical trials isn’t just about cutting costs—it can directly impact:
- Time to market: Faster trials mean earlier access for patients and a competitive edge
- Trial costs: Every month saved reduces spending on sites, personnel, and vendor fees
- Regulatory momentum: Delays in one phase often ripple into future milestones
Efficient trials benefit everyone: patients, investigators, sponsors, and regulators.
Top Bottlenecks That Slow Down Clinical Trials
Identifying the most common sources of delay helps teams focus on areas with the highest impact:
- Protocol complexity: Overly detailed or unclear protocols lead to amendments, confusion, and deviations
- Manual processes: Data transcription, paper-based workflows, or email-based collaboration increase delays and errors
- Site startup delays: Contracting, IRB approvals, and training are often sequential instead of parallel
- Slow query resolution: Data issues pile up when there’s no real-time system for review and response
- Lack of visibility: Sponsors and CROs struggle to track progress without centralized dashboards
Study Design and Planning Efficiencies
Speed starts with better planning:
- Use adaptive designs or seamless Phase 1/2 trials to eliminate transition delays
- Involve operations and data teams early to ensure protocols are feasible and unambiguous
- Choose high-performing sites based on past performance and access to the target population
- Pre-write templates for CRFs and eConsent workflows to accelerate system setup
Startup Acceleration Tactics
The study startup phase is full of avoidable friction:
- Use standardized site contracts to cut negotiation time
- Submit to a central IRB when possible to avoid site-by-site delays
- Combine feasibility, qualification, and training into one visit or digital process
- Automate document collection with a CTMS-integrated checklist
- Track startup tasks in real time with dashboards and automated alerts
Enrollment and Engagement Optimization
Faster recruitment requires scalable systems and patient-centric approaches:
- Leverage EHR data and registry platforms for rapid pre-screening
- Use digital ads with embedded eligibility questions to drive traffic to study pages
- Offer eConsent and remote onboarding to eliminate unnecessary site visits
- Provide telehealth or mobile visits to widen geographic eligibility
Smarter Data Collection and Monitoring
Slow data means slow decisions. Modernize how you capture and review information:
- Use EDC systems with real-time edit checks and auto-coding
- Implement remote monitoring supported by risk-based monitoring (RBM) plans
- Replace paper PROs with ePRO apps to eliminate transcription and late entries
- Automate query routing and escalation based on data source, site, and priority
Closeout Acceleration
Don’t wait until the end to clean your data or reconcile your documents:
- Conduct rolling data cleaning throughout the study
- Pre-schedule database lock with clear ownership of final steps
- Use eTMF systems with version control and document indexing to stay audit-ready
- Reconcile SAE reports, queries, and logs weekly instead of in bulk at the end
Technology That Speeds Up Trials
Modern platforms reduce handoffs, automate routine work, and improve transparency:
- EDC platforms with drag-and-drop builders and edit check automation
- CTMS software to track site performance and milestone completion
- eTMF systems for inspection readiness and version control
- eConsent and ePRO tools for participant convenience
- Integrated dashboards for sponsor-CRO visibility and issue escalation
Key Metrics to Track Time Savings
- Time from site selection to activation
- Time to first patient enrolled (FPI)
- Duration of query resolution cycles
- Total time from last patient visit to database lock
- Number of protocol amendments and time to implement
Real-World Examples
- A mid-sized biotech reduced study startup timelines by 30% using central IRBs and contract templates
- A decentralized rare disease trial enrolled its first patient in 10 days using a registry-based pre-screening campaign
- A global oncology study implemented remote RBM and reduced site monitoring costs by 40% without impacting data quality
Key Takeaways
- Time savings require alignment between protocol design, site operations, data systems, and sponsor oversight
- The biggest gains come from digitizing manual processes and eliminating redundant tasks
- With the right tools and planning, you can shave months off your trial timeline and stay ahead of the curve
Frequently Asked Questions (FAQs)
1. What’s the average timeline for a clinical trial?
It varies by phase and indication. Phase 2 and 3 trials typically last 1.5–3 years.
2. Can automation really reduce trial duration?
Yes—automated workflows reduce errors, minimize rework, and accelerate decision-making.
3. What’s the fastest way to improve site startup times?
Use central IRBs, contract templates, and automated document workflows.
4. How do decentralized trials affect timelines?
They can accelerate enrollment and improve retention but may require upfront planning to integrate home health or telehealth workflows.