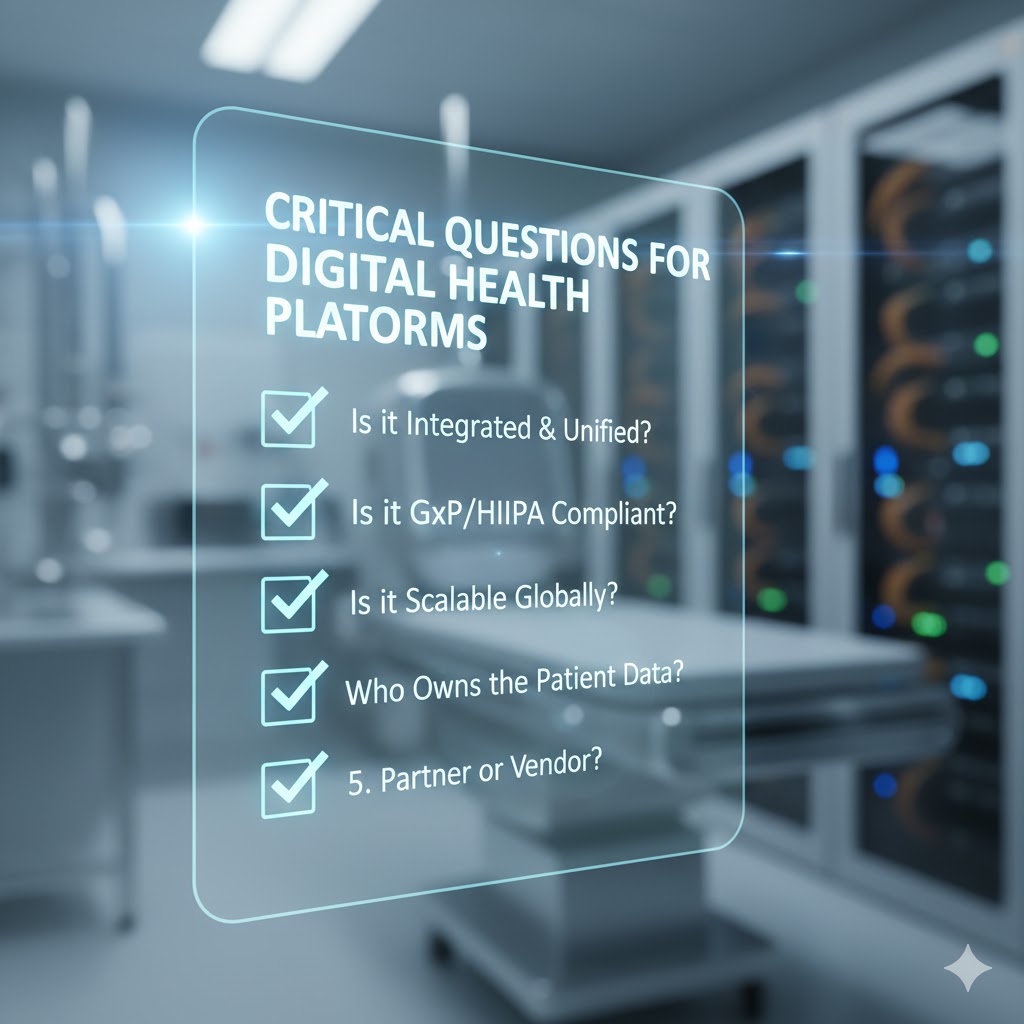
Top EDC Software Systems
In an era increasingly dominated by digitalization, data has emerged as the backbone of successful decision-making in diverse sectors, from healthcare and pharmaceuticals to social sciences and market research. One essential tool facilitating the transition from manual data collection to a seamless, digitized paradigm is Electronic Data Capture (EDC) software. With EDC, we're witnessing a radical transformation in how data is collected, stored, shared, and managed.
The last few years have seen the proliferation of a multitude of EDC software solutions, each aimed at streamlining and enhancing the efficiency of data acquisition, ensuring data integrity, and promoting real-time data sharing and collaboration. From cloud-based systems and mobile-friendly interfaces to AI-enabled analytics and stringent security measures, these software solutions are revolutionizing our approach towards data handling.
This article will delve into the world of EDC software available today. We aim to provide an in-depth overview of the leading tools in the market, their unique features, applicability across various industries, and how they cater to different business needs. Whether you're a professional seeking to upgrade your data collection methods or a business leader aiming to enhance operational efficiency, this exploration will provide a comprehensive understanding of the contemporary EDC software landscape.
1. Mahalo Health
Mahalo Health EDC is a groundbreaking solution that dramatically transforms the landscape of clinical trial management. With its comprehensive features and intuitive interface, Mahalo Health EDC empowers researchers and trial coordinators to streamline data collection, optimize patient engagement, ensure data security, and accelerate the development of life-saving interventions.
Mahalo Health EDC software encompasses a centralized hub that seamlessly integrates data collection, patient management, and study administration. By automating administrative tasks and providing real-time access to data, this platform enables researchers to efficiently track and analyze patient information while fostering collaboration among teams. The software's commitment to enhancing patient engagement and compliance is exemplified through remote patient monitoring, patient-reported outcomes, and educational materials, ultimately improving the accuracy and reliability of trial results.

Data security and compliance are paramount in clinical trials, and Mahalo Health EDC software addresses these concerns with utmost diligence. The platform adheres to stringent security standards, including HIPAA and GDPR, ensuring robust data encryption, access controls, and comprehensive audit trails. This commitment to data privacy instills confidence among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies.
Researchers can expedite the clinical trial process by leveraging Mahalo Health EDC software and accelerate the development of new treatments, therapies, and medical devices. The platform's agile functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, the data collected through Mahalo Health EDC contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes.
Furthermore, Mahalo Health EDC software facilitates the integration of real-world data and electronic health records (EHRs), expanding the scope of post-marketing surveillance and pharmacovigilance. These capabilities allow for analyzing larger datasets and enhance drug safety monitoring, contributing to regulatory compliance practices.
All in all, Mahalo Health EDC software is transforming clinical trial management practices by simplifying data collection, enhancing patient engagement, ensuring data security, and accelerating the development of life-saving interventions. Its intuitive interface and comprehensive features empower researchers and trial coordinators to conduct studies more efficiently, improving healthcare outcomes and better-informed treatment decisions. With Mahalo Health EDC, the future of clinical trials shows more promise than ever.
2. Medidata Rave
Medidata Rave EDC, a trailblazing electronic data capture (EDC) software platform, has transformed the landscape of clinical trial management and healthcare services. By providing a comprehensive and integrated system for data collection, analysis, and management, Medidata Rave has introduced significant benefits for researchers and patients alike.
At the core of Medidata Rave EDC lies the ability to streamline the clinical trial process and simplify data collection. By enabling researchers to capture and record patient information electronically, the software eliminates the need for outdated paper-based processes, reducing the likelihood of errors. Additionally, Medidata Rave centralizes data securely, promoting collaboration among research teams and enabling efficient data management.
The platform offers advanced functionalities for data analysis, reporting, and real-time access to trial data. Researchers can easily monitor the progress of their studies and make informed decisions using the platform's built-in analytics tools. The ability to perform complex data analyses, identify trends, and generate comprehensive reports effortlessly enhances the overall efficiency of clinical trials. Furthermore, Medidata Rave EDC seamlessly integrates with other healthcare systems, such as electronic health records (EHRs), laboratory information management systems (LIMS), and imaging systems. This interoperability allows for data sharing and provides a holistic view of patient health, improving patient care and outcomes.
Medidata Rave EDC also prioritizes patient engagement and participation in clinical trials. With user-friendly interfaces, mobile accessibility, and multilingual support, the platform makes it easier for patients to provide their data and participate in research studies. This accessibility contributes to a more diverse participant pool, ultimately improving the generalizability of trial results and enabling better-informed healthcare decisions and more effective treatments.
To conclude, Medidata Rave EDC is an invaluable software platform that has modernized clinical trial management and healthcare services. Its ability to streamline data capture, facilitate advanced analytics, promote interoperability, and enhance patient engagement makes it an essential tool in clinical research. By leveraging the capabilities of Medidata Rave EDC, researchers can conduct more efficient and effective trials, leading to improved healthcare outcomes for patients.
3. Oracle Clinical
Oracle Clinical’s EDC is a determinative in the realm of clinical trials. This robust software platform empowers researchers and trial coordinators with a comprehensive suite of tools to streamline the entire trial process, from data collection to study administration. With its user-friendly interface and powerful capabilities, Oracle Clinical EDC revolutionizes clinical trials.
One of the standout features of Oracle Clinical’s EDC is its ability to enhance patient engagement and compliance. Researchers can effortlessly collect data and ensure participants remain engaged throughout the trial by leveraging remote patient monitoring and patient-reported outcomes. Additionally, the platform provides educational materials to foster participant understanding, resulting in improved patient experience and satisfaction. Oracle Clinical’s EDC understands that successful trial completion hinges on engaging and retaining participants, ultimately generating reliable and accurate data.
Data security and compliance are paramount in clinical trials, and Oracle Clinical’s EDC excels in this area. The platform prioritizes data privacy and adheres to strict regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, Oracle Clinical’s EDC ensures that sensitive patient information remains secure. This commitment to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
Furthermore, Oracle Clinical’s EDC accelerates the development of new treatments, therapies, and medical devices. The platform contributes to faster approvals and the availability of life-saving interventions by streamlining the research process and enabling more efficient study conduct. The data collected through Oracle Clinical’s EDC also fuels evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. Moreover, the platform facilitates the integration of real-world data and electronic health records, allowing for comprehensive analysis and post-marketing surveillance.
Undeniably, Oracle Clinical’s EDC is a transformative software platform that propels clinical trials into the future. Its intuitive interface, patient engagement focus, and data security commitment make it an indispensable tool for researchers and trial coordinators. By leveraging the power of Oracle Clinical’s EDC, the healthcare industry can conduct more efficient trials, generate reliable data, and ultimately improve patient care and outcomes.
4. Veeva Vault EDC
Veeva Vault EDC is a leading-edge software platform that has revolutionized the landscape of clinical trial management. By providing a comprehensive and intuitive system for electronic data capture (EDC) in clinical trials, Veeva Vault EDC has redefined how researchers collect, analyze, and manage critical trial data, delivering significant benefits to research teams and patients.
With Veeva Vault EDC, researchers can streamline the entire clinical trial process, eliminating the burdensome and error-prone paper-based data collection methods. This cloud-based platform enables efficient and accurate capture of patient information, saving valuable time and resources. By centralizing data in a secure and standardized format, Veeva Vault EDC promotes seamless collaboration among research teams, ensuring everyone can access real-time data and enabling prompt decision-making.
One of the standout features of Veeva Vault EDC is its advanced analytics capabilities. Researchers can leverage powerful built-in analytics tools to perform complex data analyses, identify trends, and generate comprehensive reports effortlessly. This empowers them to gain deep insights into their studies' progress, expedite data-driven decision-making, and ultimately enhance the overall efficiency of clinical trials. Furthermore, Veeva Vault EDC seamlessly integrates with other healthcare systems, such as electronic health records (EHRs), enabling a holistic view of patient health. This interoperability facilitates data sharing and improves patient care and outcomes by providing healthcare providers real-time access to patient data for informed decision-making and personalized treatment plans.
In addition to its robust functionality, Veeva Vault EDC prioritizes data privacy and security. The platform adheres to stringent regulatory standards, ensuring compliance with HIPAA and GDPR requirements. With comprehensive data encryption, access controls, and audit trails, Veeva Vault EDC instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
In summary, Veeva Vault EDC is a trendsetter in clinical trial management. Its ability to simplify data capture, empower advanced analytics, promote interoperability, and prioritize data security makes it an indispensable tool for researchers. By leveraging the power of Veeva Vault EDC, research teams can conduct trials more efficiently, leading to faster approvals and the development of life-saving interventions. This innovative software platform contributes to evidence-based medicine, enabling informed treatment decisions and improved patient outcomes.
5. IBM Clinical Development
IBM Clinical Development is a cutting-edge EDC software that has recast the way clinical trials are conducted. This comprehensive platform provides researchers and trial coordinators with a centralized hub for data collection, patient management, and study administration. By leveraging the power of cloud technology, IBM Clinical Development simplifies the entire trial process, enabling seamless collaboration and real-time data access.
One of the critical advantages of IBM Clinical Development is its focus on patient engagement and compliance. Recognizing that successful trial completion relies on engaged and retained participants, the platform incorporates tools to enhance the patient experience. From remote patient monitoring to simplified data collection through patient-reported outcomes, the software streamlines the data collection process while ensuring participant understanding. By prioritizing patient satisfaction, IBM Clinical Development increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and IBM Clinical Development understands this. The platform is designed to prioritize data privacy and complies with regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure the utmost security and instill confidence among stakeholders. This commitment to data security facilitates seamless collaboration between researchers, sponsors, and regulatory bodies, driving progress in medical research.
IBM Clinical Development is a software platform and a significant leap forward in clinical trial management. Streamlining the research process accelerates the development of new treatments, therapies, and medical devices. The platform's advanced functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through IBM Clinical Development contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. Integrating real-world data and electronic health records further enhances post-marketing surveillance and pharmacovigilance, contributing to drug safety monitoring and regulatory compliance practices.
IBM Clinical Development's EDC software is definitely a decisive technological innovation in clinical trials. Its cloud-based platform simplifies data collection, enhances patient engagement, ensures data security and compliance, and accelerates medical advancements. By embracing this innovative solution, researchers and healthcare providers can drive progress in the field, delivering better healthcare outcomes for patients worldwide.
6. OpenClinica
OpenClinica, a leading EDC software platform, has reorganized how clinical trials are conducted, propelling the field forward with its comprehensive features and user-friendly interface. Researchers and trial coordinators can now access a centralized hub that simplifies data collection, patient management, and study administration. With OpenClinica, the cumbersome processes of the past are replaced with streamlined workflows, leading to increased efficiency and accuracy.
One of the critical strengths of OpenClinica is its focus on engaging and retaining participants in clinical trials. Recognizing the importance of generating reliable data, the platform employs tools that enhance patient engagement and compliance. Remote patient monitoring, patient-reported outcomes, and educational materials are some features that foster participant understanding and satisfaction. By prioritizing patient experience, OpenClinica ensures the smooth functioning of trial components, ultimately improving the likelihood of study completion and the accuracy of trial results.
Security and compliance are paramount in clinical trials that involves sensitive patient data. OpenClinica understands this, and the platform adheres to strict data privacy regulations, including HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, OpenClinica instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies. The platform's commitment to data security ensures that sensitive information remains protected, fostering trust and facilitating the advancement of medical research.
OpenClinica's impact extends beyond the realm of clinical trials. The platform is crucial in advancing healthcare by streamlining the research process and accelerating the development of new treatments, therapies, and medical devices. Its functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through OpenClinica contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. With its ability to integrate real-world data and electronic health records, OpenClinica facilitates post-marketing surveillance and pharmacovigilance, enhancing drug safety monitoring and regulatory compliance practices.
OpenClinica's EDC software has veered the field of clinical trials towards positive transformation by providing a comprehensive platform that simplifies data collection, enhances participant engagement, ensures data security, and accelerates the development of new medical interventions. Its impact extends beyond research, empowering healthcare providers, and improving patient outcomes. OpenClinica has genuinely transformed the landscape of clinical trials, bringing us one step closer to a healthier future.
7. REDCap
REDCap (Research Electronic Data Capture) is an exemplary EDC software that restyles the management of clinical trials. With its comprehensive features and user-friendly interface, REDCap empowers researchers and trial coordinators to streamline data collection, analysis, and study administration. This cloud-based platform is a centralized hub for all trial-related activities, providing real-time access and seamless collaboration.
REDCap focuses on patient engagement and compliance. Recognizing successful trial completion hinges on participant involvement; REDCap offers tools to enhance patient experience and understanding. From remote patient monitoring to simplified data collection through patient-reported outcomes, REDCap facilitates active patient participation. This software platform significantly generates reliable trial results by fostering engagement and providing educational materials.
Data security and compliance are of utmost importance in clinical trials, and REDCap excels in these areas. The platform prioritizes data privacy and adheres to strict regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure that sensitive patient data remains secure throughout the trial. This commitment to data protection instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
REDCap is a difference-making EDC software that transforms the landscape of clinical trials. Its user-friendly interface, comprehensive functionalities, and focus on patient engagement make it an invaluable tool for researchers and trial coordinators. By streamlining the research process, accelerating treatment development, and ensuring data security and compliance, REDCap paves the way for faster approvals, evidence-based medicine, and improved patient outcomes. Embracing the capabilities of REDCap empowers researchers to conduct more efficient and effective trials, ultimately advancing healthcare for the benefit of all.
8. Castor EDC
Castor EDC is a revolutionary software platform transforming the landscape of clinical trials. With its robust features and user-friendly interface, Castor EDC simplifies the entire process, from data collection to study management, empowering researchers and coordinators to conduct trials with unprecedented efficiency.
At the core of Castor EDC are its robust data collection capabilities. Researchers can effortlessly capture patient information electronically, eliminating the burdensome paperwork of traditional methods. This not only saves valuable time and resources but also minimizes the risk of errors and ensures the accuracy of data. With Castor EDC, data management becomes a breeze, as it centralizes all information in a secure and standardized format, promoting seamless collaboration among research teams.
Castor EDC goes beyond data collection and offers advanced data analysis and reporting functionalities. Researchers have real-time access to trial data, enabling them to monitor the progress of their studies and make informed decisions promptly. The platform's built-in analytics tools empower researchers to perform complex analyses, identify trends, and generate comprehensive reports effortlessly. This capability enhances the overall efficiency of clinical trials, accelerating the generation of valuable insights and expediting the development of life-saving interventions.
One of the critical strengths of Castor EDC lies in its commitment to patient engagement. The platform provides user-friendly interfaces, mobile accessibility, and multilingual support, ensuring that participants can easily offer their data and engage in research studies. By breaking down barriers to participation, Castor EDC facilitates a more diverse participant pool, leading to more inclusive and representative trial results. This, in turn, enables healthcare providers to make better-informed decisions, develop personalized treatment plans, and deliver more effective care.
Castor EDC is another ace in software systems for clinical trials. Its comprehensive features streamline data collection, empower researchers with powerful analysis tools, and enhances patient engagement. By leveraging the capabilities of Castor EDC, researchers can conduct trials with unprecedented efficiency, ultimately leading to improved healthcare outcomes and the development of innovative treatments and therapies. Castor EDC is reshaping how clinical trials are conducted, setting a new standard for EDC software in the industry.
9. Medrio
Medrio EDC, too is a watermark software in the realm of running clinical trials. With its user-friendly interface and comprehensive functionality, this EDC software empowers researchers and trial coordinators to streamline the entire process from data collection to analysis. By leveraging the power of cloud technology, Medrio EDC provides a centralized hub for managing patient data, study protocols, and administrative tasks, all in real time.
Engaging and retaining participants in clinical trials is essential for generating reliable data, and Medrio EDC understands this critical aspect. Through innovative tools, such as remote patient monitoring and patient-reported outcomes, the software platform enhances patient engagement and compliance. By simplifying data collection and providing educational materials, Medrio EDC ensures participants have a smooth and informed experience, ultimately improving study completion rates and the accuracy of trial results.
Data security and compliance are paramount in clinical trials, and Medrio EDC takes this responsibility seriously. With robust data encryption, access controls, and comprehensive audit trails, the platform prioritizes data privacy while complying with regulations like HIPAA and GDPR. This commitment to security fosters trusts among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
Medrio EDC doesn't just revolutionize the trial process; it also accelerates the development of life-saving treatments, therapies, and medical devices. By streamlining research and improving efficiency, this software platform enables faster approvals and the availability of interventions that can transform patients' lives. Furthermore, the data collected through Medrio EDC contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes.
Medrio EDC is a powerful software platform revolutionizing clinical trial management. With its user-friendly interface, advanced data analysis capabilities, and emphasis on patient engagement, Medrio EDC empowers researchers to conduct trials more efficiently and effectively. Medrio EDC fosters stakeholder collaboration and trust by prioritizing data security and compliance. Ultimately, this software platform accelerates medical advancements and improves patient care worldwide.
10. TrialMaster
"TrialMaster, the leading EDC software, revolutionizes the landscape of clinical trials by providing researchers with a powerful tool to streamline the entire process. This comprehensive platform simplifies data collection, enhances patient engagement, ensures data security, and accelerates the development of life-saving interventions. TrialMaster sets the stage for successful clinical trials and transforms how researchers gather and analyze critical data.
With TrialMaster, researchers access a centralized hub that seamlessly manages patient data, study protocols, and administrative tasks. The intuitive interface fosters collaboration and real-time data access, enabling researchers and trial coordinators to track and analyze patient data efficiently. By leveraging advanced patient engagement and compliance tools, TrialMaster simplifies data collection through patient-reported outcomes and facilitates remote patient monitoring. This commitment to enhancing the patient experience results in higher participant retention rates, ultimately leading to more reliable and accurate trial results.
Data security and compliance are paramount in clinical trials, and TrialMaster takes these concerns seriously. The platform adheres to the strictest data privacy standards, including HIPAA and GDPR compliance. Robust data encryption, access controls, and comprehensive audit trails protect sensitive patient information. Stakeholders can confidently collaborate, knowing that TrialMaster prioritizes data security and meets regulatory requirements.
By offering seamless integration with real-world data and electronic health records (EHRs), TrialMaster contributes to post-marketing surveillance, pharmacovigilance, and evidence-based medicine. The platform empowers researchers to analyze larger datasets, promoting drug safety monitoring and regulatory compliance practices. Furthermore, TrialMaster expedites the development of new treatments, therapies, and medical devices by streamlining the research process, obtaining faster approvals, and improving patient outcomes.
TrialMaster is a pivotal EDC software that drives innovation and efficiency in clinical trials. Its comprehensive functionalities, commitment to data security, and focus on patient engagement make it an indispensable tool for researchers. With TrialMaster, the future of clinical trials is brighter, as it empowers researchers to generate reliable data and deliver life-changing interventions with greater speed and accuracy."
11. Formedix
Formedix, the pioneering leader in EDC software solutions, has emerged as a difference-maker in the clinical trial industry. Their cutting-edge platform provides researchers and trial coordinators with an unparalleled toolkit to streamline and optimize the trial process. With Formedix, the complexities of data collection, patient management, and study administration are effortlessly transformed into seamless operations, empowering researchers to focus on what truly matters: advancing medical knowledge and improving patient outcomes.
At the heart of Formedix's success lies its robust EDC software, which acts as the central nervous system of clinical trials. The platform's user-friendly interface and intuitive design make it a breeze for researchers to track and analyze patient data, manage study protocols, and automate administrative tasks. By eliminating traditional paper-based processes' laborious and error-prone nature, Formedix's EDC software saves valuable time and resources while significantly enhancing data accuracy and reliability.
Formedix's unwavering commitment to patient engagement and compliance sets it apart from its competitors. Recognizing that successful trial completion hinges on effective participant involvement, Formedix's EDC software leverages innovative tools to foster engagement and improve the trial experience. Through remote patient monitoring, simplified data collection via patient-reported outcomes, and educational materials, Formedix empowers participants and facilitates their understanding of the trial process. Formedix ensures higher retention rates by prioritizing patient satisfaction and generating robust and reliable data for better-informed medical decisions.
Security and compliance are paramount when dealing with sensitive patient data, and Formedix's EDC software excels in this area. The platform adheres to the strictest data privacy regulations, including HIPAA and GDPR, ensuring the highest level of protection for patient information. Robust data encryption, granular access controls, and comprehensive audit trails provide stakeholders with the peace of mind to collaborate seamlessly with researchers, sponsors, and regulatory bodies. With Formedix, data security is not just a feature—it's a core value that instills trust and confidence among all stakeholders involved.
Formedix's innovative EDC software platform is not just a tool; it's a catalyst for transforming the clinical trial landscape. By streamlining research processes, accelerating treatment development, and improving patient outcomes, Formedix revolutionizes the way trials are conducted. The platform's advanced functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, the data collected through Formedix's EDC software contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and revolutionizing patient care.
In a rapidly evolving world where the integration of real-world data and electronic health records is becoming increasingly crucial, Formedix's EDC software rises to the challenge. The platform seamlessly integrates with other healthcare systems, such as electronic health records (EHRs), laboratory information management systems (LIMS), and imaging systems. This enables comprehensive data sharing and a holistic view of patient health. This interoperability enhances patient care and outcomes and supports post-marketing surveillance and pharmacovigilance, bolstering drug safety monitoring and regulatory compliance practices.
Formedix's EDC software has emerged as a radical turning point in clinical trials. By simplifying data capture, promoting collaboration, ensuring data security, and enhancing patient engagement, Formedix empowers researchers to conduct more efficient and effective trials. Through its groundbreaking platform, Formedix paves the way for groundbreaking medical discoveries and ultimately improves the lives of countless patients worldwide.
12. ArisGlobal agCapture
agCapture by ArisGlobal is a special EDC software that mutates the process of running clinical trials. Designed to simplify and streamline the intricate journey of research studies, agCapture offers a comprehensive suite of features for efficient data collection, management, and analysis, empowering researchers and trial coordinators to achieve optimal results.
One of the standout features of agCapture is its user-friendly interface, which allows for seamless collaboration and real-time data access. This intuitive design ensures that researchers and coordinators can effortlessly track and analyze patient data, manage study protocols, and automate administrative tasks, all from a centralized hub. By eliminating unnecessary complexities, agCapture empowers research teams to focus on what truly matters – generating reliable data and driving medical advancements.
EDC software like agCapture plays a crucial role in enhancing patient engagement and compliance, which is paramount for the successful completion of clinical trials. With tools dedicated to improving the participant experience, agCapture facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By putting the patient at the center of the process, agCapture increases the likelihood of study completion while improving the accuracy and reliability of trial results.
In clinical trials, data security, and compliance are of paramount importance. With agCapture, ArisGlobal emphasizes protecting sensitive patient data, ensuring HIPAA and GDPR compliance. The platform offers robust data encryption, access controls, and comprehensive audit trails, fostering trust and confidence among stakeholders. By prioritizing data privacy, agCapture enables seamless collaboration between researchers, sponsors, and regulatory bodies, securely and ethically driving medical science's advancement.
agCapture from ArisGlobal is an exceptional EDC software that empowers research teams to conduct clinical trials efficiently and confidently. By streamlining the research process and prioritizing patient engagement, agCapture accelerates the development of life-saving interventions while maintaining the highest data security and compliance standards. With its comprehensive suite of features, agCapture is poised to reshape the landscape of clinical trials, contributing to evidence-based medicine and improving patient outcomes for years.
13. Dacima Clinical Suite
Dacima Clinical Suite, an innovative EDC software platform, has transformed how clinical trials are managed by providing researchers and trial coordinators with a comprehensive and streamlined solution. With its user-friendly interface and robust functionalities, Dacima Clinical Suite simplifies the entire trial process, from data collection to patient management and study administration. By optimizing efficiency and enhancing collaboration, this platform enables researchers to generate reliable data and expedite the development of life-saving interventions.
One of the critical strengths of Dacima Clinical Suite lies in its focus on patient engagement and compliance. Recognizing that participant retention is essential for successful trial completion, Dacima integrates tools that enhance patient experience and foster understanding. Through remote patient monitoring and patient-reported outcomes, the software facilitates seamless data collection while educational materials empower participants to engage in the trial process actively. By prioritizing patient satisfaction, Dacima Clinical Suite ensures a higher likelihood of study completion and enhances the accuracy and reliability of trial results.
Security and compliance are prime importance in clinical trials, and Dacima Clinical Suite is designed with these considerations in mind. The platform adheres to the highest standards of data privacy, complying with regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails safeguard sensitive patient information, fostering trust among stakeholders. This commitment to data security enables seamless collaboration between researchers, sponsors, and regulatory bodies, ensuring that the integrity of clinical trial data is upheld.
The impact of Dacima Clinical Suite extends beyond the realm of individual trials. By streamlining the research process and accelerating the development of new treatments, therapies, and medical devices, this software platform contributes to evidence-based medicine and improves patient outcomes. Moreover, Dacima Clinical Suite facilitates the integration of real-world data and electronic health records, enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance. With its wide-ranging capabilities, Dacima Clinical Suite catalyzes innovation in clinical research, empowering researchers to make informed decisions and revolutionize healthcare practices.
Dacima Clinical Suite is a cutting-edge EDC software platform that propels the field of clinical trials forward. Dacima Clinical Suite empowers researchers to conduct more efficient and effective trials by prioritizing patient engagement, ensuring data security, and facilitating collaboration. Through its impact on trial completion rates, data accuracy, and overall research efficiency, Dacima Clinical Suite contributes to advancing evidence-based medicine and improving patient care on a global scale through its impact on trial completion rates, data accuracy, and overall research efficiency.
14. DCify
In clinical trials, the effective management of data and the seamless coordination of various stakeholders are paramount. This is where EDC software like Edcify steps in, providing researchers and trial coordinators with a comprehensive platform to revolutionize clinical trials. Edcify is a cutting-edge solution that streamlines the research process, enhances data accuracy, and fosters collaboration among research teams.
With Edcify, researchers can bid farewell to outdated paper-based processes and embrace the power of electronic data capture. This agile platform enables collecting and recording patient information electronically, eliminating errors, saving valuable time, and significantly improving data accuracy. Edcify's intuitive interface simplifies data management and offers real-time access to trial data, empowering researchers to monitor progress and make informed decisions swiftly. Its robust analytics tools enable complex data analyses and generate comprehensive reports effortlessly, driving quicker data-driven insights and overall trial efficiency.
EDC software like Edcify transforms how clinical trials are conducted and paves the way for better patient care. By seamlessly integrating with electronic health records (EHRs) and other healthcare systems, Edcify facilitates a holistic view of patient health. This interoperability enhances collaboration among healthcare providers, promoting informed decision-making, personalized treatment plans, and efficient care coordination. Moreover, Edcify's user-friendly interfaces, mobile accessibility, and multilingual support enhance patient engagement and participation in clinical trials. This accessibility leads to a more diverse participant pool, ultimately generating trial results that are more representative of real-world patient populations.
In an era where data security and compliance top all considerations, Edcify is a trusted guardian of sensitive patient information. The platform prioritizes data privacy, employing robust encryption, access controls, and comprehensive audit trails. Edcify adheres to stringent regulatory standards, providing stakeholders the confidence and peace of mind to collaborate seamlessly.
Edcify is more than just a software platform; it is a catalyst for transforming clinical trials and driving advancements in healthcare. By streamlining data capture, enhancing collaboration, and improving patient engagement, Edcify empowers researchers to conduct more efficient and effective trials. This accelerates the development of new treatments and improves healthcare outcomes for patients worldwide. With Edcify leading the way, the future of clinical research looks brighter than ever.
15. Clincase
Clinicase is an EDC software platform that makes over how clinical trials are conducted and managed. With its comprehensive suite of features and user-friendly interface, Clinicase simplifies the clinical trial process, allowing researchers and trial coordinators to streamline data collection, patient management, and study administration seamlessly.
One of the critical strengths of Clinicase is its focus on enhancing patient engagement and compliance. The platform offers tools that facilitate remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By prioritizing the patient experience and satisfaction, Clinicase ensures that participants remain engaged and motivated throughout the trial, leading to higher retention rates and more reliable data.
Security and compliance heads the list of essentialities in clinical trials, and Clinicase understands this. The platform prioritizes data privacy and complies with strict regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, Clinicase provides a secure environment for sensitive patient information. This commitment to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
By leveraging the power of EDC software like Clinicase, researchers can accelerate the development of new treatments, therapies, and medical devices. The platform streamlines the research process, enabling more efficient study conduct, faster approvals, and the availability of life-saving interventions. Moreover, the data collected through Clinicase contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes.
Clinicase is a breakthrough software in the field of clinical trials. Its advanced functionalities, patient-centric approach, and commitment to data security make it an indispensable tool for researchers and trial coordinators. By harnessing the power of EDC software like Clinicase, we can advance medical research, improve patient care, and save lives.
16. OmniComm TrialMaster
OmniComm TrialMaster is a trailblazing software platform revolutionizing the landscape of clinical trials. By providing a comprehensive and robust electronic data capture (EDC) solution, TrialMaster empowers researchers and trial coordinators to streamline the entire trial process, from data collection to analysis, with unparalleled efficiency and accuracy.
At the heart of OmniComm TrialMaster lies its powerful EDC software, which enables researchers to capture, manage, and analyze patient data electronically. This eliminates the need for cumbersome paper-based processes, reducing the likelihood of errors and ensuring data accuracy. With TrialMaster, researchers can efficiently track and analyze patient data, manage study protocols, and automate administrative tasks, all within a centralized hub. The intuitive interface fosters seamless collaboration and real-time data access, facilitating a smooth and cohesive research experience.
One of the key strengths of OmniComm TrialMaster is its unwavering commitment to patient engagement and satisfaction. The software platform incorporates tools that enhance participant involvement and compliance throughout the trial. With remote patient monitoring, patient-reported outcomes, and educational materials, TrialMaster ensures that participants feel supported and informed, improving retention rates and reliable data collection. By prioritizing the patient experience, TrialMaster significantly increases the likelihood of study completion, enhancing the accuracy and reliability of trial results.
Regarding data security and compliance, OmniComm TrialMaster sets the gold standard. Recognizing the sensitivity of patient data, TrialMaster adheres to stringent privacy regulations, such as HIPAA and GDPR. The platform employs robust data encryption, comprehensive access controls, and detailed audit trails, safeguarding the integrity and confidentiality of the data. This commitment to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
OmniComm TrialMaster, with its cutting-edge EDC software, is transforming the landscape of clinical trials. By streamlining the research process, TrialMaster accelerates the development of new treatments and therapies. Its patient-centric approach improves participant engagement and satisfaction, increasing retention rates and creating more reliable data. With its unwavering commitment to data security and compliance, TrialMaster ensures the integrity and confidentiality of patient information. OmniComm TrialMaster is genuinely at the forefront of innovation, restyling how clinical trials are conducted and bringing us closer to improved healthcare outcomes.
17. BioClinica EDC
BioClinica EDC software revolutionizes clinical trials, offering a powerful platform that optimizes efficiency and data management. With its comprehensive suite of features, BioClinica EDC empowers researchers and trial coordinators to streamline their processes and achieve reliable results.
The key strength of BioClinica EDC lies in its ability to simplify data collection and analysis. By providing a centralized hub for data management, researchers can easily track and monitor patient data, ensuring accurate and up-to-date information. The platform's intuitive interface facilitates seamless collaboration, allowing multiple stakeholders to access real-time data effortlessly. With BioClinica EDC, the days of cumbersome paper-based processes are long gone, replaced by an agile and efficient digital solution.
One of the standout features of BioClinica EDC is its emphasis on data security and compliance. Recognizing the sensitive nature of patient information, BioClinica prioritizes data privacy and adheres to industry standards such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure that data remains secure throughout the entire trial process. This commitment to data security instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
Furthermore, BioClinica EDC enhances the overall quality and efficiency of clinical trials. The platform accelerates the trial process by automating administrative tasks and providing advanced analytics tools and enables researchers to make data-driven decisions promptly. Real-time access to trial data and the integration of electronic health records (EHRs) facilitate a holistic view of patient health, improving patient care and outcomes. With BioClinica EDC, researchers can focus on their core objectives and drive innovation in the field of healthcare.
BioClinica EDC software is a process-changer for clinical trials. Its comprehensive features streamline data collection, enhance collaboration, and ensure data security. By leveraging the power of BioClinica EDC, researchers and trial coordinators can optimize their processes, achieve reliable results, and contribute to advancements in healthcare. This platform is a testament to technology's transformative potential in revolutionizing how we conduct clinical trials and improve patient outcomes.
18. DataFax
DataFax, a spearheading EDC software, is transforming the landscape of clinical trials by offering a seamless and efficient data collection, management, and analysis platform. With its robust features and user-friendly interface, DataFax empowers researchers and trial coordinators to navigate the complexities of clinical research with ease and precision.
One key advantage of DataFax is its ability to enhance participant engagement and retention in clinical trials. By leveraging innovative tools, such as remote patient monitoring and patient-reported outcomes, DataFax ensures that researchers can collect accurate and timely data while simplifying the experience for participants. The platform goes beyond data collection and offers educational materials to foster participant understanding, ultimately improving patient experience and satisfaction. By optimizing the trial components, DataFax increases the likelihood of study completion and enhances the accuracy and reliability of trial results.
Security and compliance are paramount in clinical trials, and DataFax addresses these concerns comprehensively. With strict adherence to HIPAA and GDPR, DataFax prioritizes data privacy by implementing robust data encryption, access controls, and comprehensive audit trails. This commitment to safeguarding sensitive patient data instills confidence among stakeholders and enables seamless collaboration between researchers, sponsors, and regulatory bodies. By ensuring the utmost data security, DataFax creates a foundation of trust that is essential for the success of clinical trials.
DataFax is not just a software platform but a catalyst for developing life-saving interventions. By streamlining the research process and accelerating the approval of new treatments, therapies, and medical devices, DataFax contributes to advancing evidence-based medicine. The platform's functionalities empower researchers to conduct studies more efficiently, leading to faster approvals and better patient outcomes. Furthermore, DataFax facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance. This data-driven approach contributes to drug safety monitoring and regulatory compliance practices, further strengthening the impact of clinical trials.
DataFax revolutionizes the landscape of clinical trials through its powerful EDC software. By streamlining data collection, enhancing participant engagement, ensuring data security, and enabling data-driven insights, DataFax empowers researchers to conduct more efficient and effective trials. With its impact extending beyond the research realm, DataFax paves the way for better healthcare outcomes and advancements in evidence-based medicine.
19. InformEDC
InformEDC, a cutting-edge electronic data capture (EDC) software, is transforming the landscape of clinical trials. With its comprehensive suite of features and intuitive interface, InformEDC simplifies the entire trial process, empowering researchers and coordinators to efficiently track data, manage protocols, and automate administrative tasks. By leveraging the power of InformEDC, clinical trials can achieve unprecedented levels of efficiency and reliability.
Engaging and retaining participants is the cornerstone of successful clinical trials, and InformEDC excels in this crucial aspect. The software employs a range of tools to enhance patient engagement and compliance. Researchers can collect data seamlessly through remote patient monitoring and patient-reported outcomes, while educational materials foster participant understanding. InformEDC's dedication to improving the patient experience ensures smoother trial components, ultimately increasing study completion rates and enhancing the accuracy and reliability of trial results.
Security and compliance are paramount in clinical trials, considering the sensitive nature of patient data. InformEDC prioritizes data privacy and rigorously complies with industry regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails safeguard patient information, fostering trust and enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
The impact of InformEDC extends far beyond the realm of individual trials. By streamlining the research process, InformEDC accelerates the development of new treatments, therapies, and medical devices. Its advanced functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through InformEDC contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes.
InformEDC's influence is not limited to the confines of clinical trials. Its seamless integration with real-world data and electronic health records (EHRs) enables the analysis of large datasets for post-marketing surveillance and pharmacovigilance. By contributing to drug safety monitoring and regulatory compliance practices, InformEDC ensures the continued well-being of patients even after the trials have concluded.
InformEDC is reorganizing clinical trials with its comprehensive features, seamless user experience, and unwavering commitment to data security. By empowering researchers, engaging participants, and advancing healthcare services, InformEDC paves the way for a future of more efficient, reliable, and impactful clinical research. With InformEDC at the forefront, the potential for innovation in healthcare is limitless.
20. Anju eClinical
Anju eClinical is an innovative EDC software revamping the landscape of clinical trials. With its comprehensive suite of features, Anju eClinical empowers researchers and trial coordinators to streamline the trial process, enhance patient engagement, and ensure data integrity.
Anju eClinical serves as a centralized hub for seamless data collection, patient management, and study administration. By harnessing the power of this platform, researchers can efficiently track and analyze patient data, manage study protocols, and automate administrative tasks. The intuitive interface fosters collaboration and enables real-time data access, empowering research teams to make informed decisions and drive study progress.
Patient engagement and retention are critical factors in the success of any clinical trial. Anju eClinical recognizes this and offers tools to enhance participant compliance and engagement. Remote patient monitoring capabilities facilitate real-time data collection, while patient-reported outcomes simplify data capture. Furthermore, the platform provides educational materials to foster participant understanding, ensuring a positive patient experience. By optimizing trial components, Anju eClinical significantly improves the likelihood of study completion and enhances the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and Anju eClinical prioritizes these aspects. The platform adheres to rigorous data privacy standards such as HIPAA and GDPR. Robust data encryption, comprehensive access controls, and detailed audit trails safeguard sensitive patient information. This commitment to data security fosters trust among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies.
Anju eClinical's software platform represents a transformative solution for the successful completion of clinical trials. By streamlining the research process, it accelerates the development of new treatments, therapies, and medical devices. Researchers benefit from enhanced efficiency, leading to faster approvals and the availability of life-saving interventions. Moreover, the data collected through Anju eClinical contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes.
Furthermore, the platform facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance. This integration enhances drug safety monitoring and supports regulatory compliance practices, contributing to the overall advancement of healthcare services.
Anju eClinical's EDC software is a big swing in clinical trials. Its comprehensive features, patient engagement focus, and data security commitment make it a vital tool for researchers and trial coordinators. By leveraging Anju eClinical, they can optimize trial management, drive efficiency, and ultimately deliver better healthcare outcomes for patients worldwide.
21. ClinCapture
ClinCapture has emerged a winner in clinical trials, revolutionizing how research is conducted through its advanced electronic data capture (EDC) software. This comprehensive platform is designed to simplify and streamline the clinical trial process, empowering researchers and trial coordinators with powerful data collection, management, and analysis tools. With ClinCapture, the complexities of running clinical trials are seamlessly transformed into a smooth and efficient journey.
At the heart of ClinCapture lies its intuitive interface, which enables researchers to track and analyze patient data while managing study protocols effortlessly. The platform is a centralized data collection and patient recruitment hub through its comprehensive platform management and study administration, facilitating real-time access and seamless collaboration among team members. By leveraging the power of ClinCapture's EDC software, researchers can optimize their workflows, reduce the likelihood of errors, and enhance the accuracy and reliability of trial results.
EDC software has brought about a paradigm shift in engaging and retaining participants in clinical trials, and ClinCapture leads the way in this regard. The platform offers a range of tools to enhance patient engagement and compliance, including remote patient monitoring, patient-reported outcomes, and educational materials. By prioritizing the patient experience and satisfaction, ClinCapture ensures that participants remain actively involved throughout the trial, leading to higher completion rates and generating reliable and meaningful data.
In these technological times where data security and compliance are paramount, ClinCapture's EDC software rises to the challenge. The platform strongly focuses on data privacy and adheres to the highest industry standards, including HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails are just a few examples of the security measures implemented by ClinCapture. This commitment to data protection fosters trust among stakeholders, allowing for seamless collaboration between researchers, sponsors, and regulatory bodies.
ClinCapture's EDC software empowers researchers to navigate the complexities of clinical trials with confidence, accelerating the development of new treatments, therapies, and medical devices. The platform's capabilities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through ClinCapture contributes to evidence-based medicine, allowing healthcare providers to make informed treatment decisions and ultimately improve patient outcomes.
In addition to its impact on clinical trials, ClinCapture also facilitates the integration of real-world data and electronic health records (EHRs), opening new possibilities for post-marketing surveillance and pharmacovigilance. This integration enhances drug safety monitoring and regulatory compliance practices, driving continuous improvement and ensuring the highest standards of patient care.
ClinCapture's EDC software is reshaping the landscape of clinical trials, empowering researchers to conduct studies with greater efficiency and confidence. ClinCapture simplifies data collection, management, and analysis through its comprehensive platform, fostering collaboration and delivering reliable results. By embracing ClinCapture, the research community can embark on a new era of clinical trials, driven by innovation, efficiency, and improved patient outcomes.
22. MACRO
Clinical trials are complex endeavors that require meticulous planning, efficient data management, and seamless collaboration among various stakeholders. EDC software has revolutionized clinical trials, offering a streamlined approach to data capture, analysis, and management. One such platform that exemplifies this transformation is Clinical Studio.
Clinical Studio is a comprehensive cloud-based platform that simplifies the clinical trial process. By offering a centralized hub for data collection, patient management, and study administration, it empowers researchers and trial coordinators to track and analyze patient data efficiently. The intuitive interface enables seamless collaboration and real-time data access, fostering a conducive environment for effective trial management.
Ensuring participant engagement and retention is crucial for generating reliable data in clinical trials, and Clinical Studio recognizes this. Through tools for enhancing patient engagement and compliance, such as remote patient monitoring and patient-reported outcomes, the platform simplifies data collection. It also provides educational materials to foster participant understanding, prioritizing patient experience and satisfaction. By optimizing trial components, Clinical Studio increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Security and compliance are paramount in clinical trials, particularly when dealing with sensitive patient data. Clinical Studio addresses these concerns by prioritizing data privacy and adhering to stringent regulations like HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, the platform instills confidence among stakeholders. This commitment to data security enables seamless collaboration between researchers, sponsors, and regulatory bodies, facilitating the advancement of medical research.
Clinical Studio is a software platform that streamlines the research process and accelerates the development of new treatments, therapies, and medical devices. By providing efficient data capture, enhanced collaboration, and improved patient engagement, it contributes to faster approvals and the availability of life-saving interventions. Additionally, the platform facilitates the integration of real-world data and electronic health records, further enhancing drug safety monitoring and regulatory compliance practices. With Clinical Studio, researchers can conduct studies more efficiently, leading to better-informed treatment decisions and improved patient outcomes.
23. MedNet Solutions iMedNet
iMedNet, developed by MedNet Solutions, definitely deserves a place in the hall of healthtech fame for its EDC software for clinical trials. This innovative platform revolutionizes how researchers and trial coordinators collect, manage, and analyze data, offering a streamlined and efficient approach to clinical trial management.
With iMedNet, the clinical trial process becomes more seamless and organized. The software provides a centralized hub where researchers can easily track patient data, manage study protocols, and automate administrative tasks. The intuitive interface facilitates team members' collaboration, allowing real-time data access and enhancing overall productivity. By simplifying these essential aspects of clinical trials, iMedNet enables researchers to focus more on the scientific aspects of their work and accelerates the development of new treatments and therapies.
A key strength of iMedNet lies in its dedication to patient engagement and satisfaction. The software incorporates tools that enhance participant experience, such as remote patient monitoring and patient-reported outcomes. Researchers can foster participant understanding and compliance by leveraging these features, resulting in more reliable data collection. iMedNet's emphasis on patient-centricity contributes to higher retention rates and ensures the successful completion of clinical trials, leading to more accurate and impactful trial results.
Data security and compliance are paramount in clinical trials; iMedNet recognizes this. The software prioritizes data privacy and adheres to industry standards such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails are implemented to safeguard sensitive patient information. By maintaining a secure environment, iMedNet instills confidence among stakeholders, including researchers, sponsors, and regulatory bodies, fostering trust and enabling smooth collaboration.
iMedNet is a groundbreaking EDC software that transforms the landscape of clinical trial management. Its comprehensive features and user-friendly interface simplify data collection, enhance patient engagement, and ensure data security. By leveraging the capabilities of iMedNet, researchers can conduct trials more efficiently, generate reliable results, and ultimately contribute to advancing healthcare and improving patient outcomes.
24. MasterControl Clinical Excellence
MasterControl Clinical Excellence is modifying the clinical trial landscape with its innovative EDC software solutions. With a comprehensive suite of tools and features, MasterControl empowers researchers and trial coordinators to streamline the entire clinical trial process, enhancing efficiency, accuracy, and collaboration.
At the heart of MasterControl Clinical Excellence is its powerful EDC software. This state-of-the-art platform provides a centralized hub for data collection, patient management, and study administration. With its intuitive interface, researchers can seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks. The real-time data access and collaboration capabilities of the EDC software enable efficient cross-functional communication, fostering synergy among research teams and accelerating trial timelines.
Patient engagement and retention are paramount for generating reliable data in clinical trials, and MasterControl Clinical Excellence excels in this aspect. Leveraging its EDC software, MasterControl offers tools to enhance patient engagement and compliance throughout the trial. It facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By prioritizing the patient experience, MasterControl ensures the smooth working of trial components, increasing the likelihood of study completion and improving the accuracy and reliability of trial results.
Security and compliance are critical in clinical trials, and MasterControl Clinical Excellence has prioritized them. The EDC software adheres to stringent data privacy regulations, including HIPAA and GDPR, ensuring the protection of sensitive patient information. Robust data encryption, access controls, and comprehensive audit trails provide a secure environment for collaboration between researchers, sponsors, and regulatory bodies. By instilling confidence among stakeholders, MasterControl Clinical Excellence enables seamless data exchange and fosters trust, driving the success of clinical trials.
MasterControl Clinical Excellence's EDC software is a radical innovation in clinical trials. By simplifying data collection, enhancing patient engagement, and ensuring data security, MasterControl empowers researchers to conduct trials more efficiently and generate high-quality data. With its comprehensive suite of tools and commitment to excellence, MasterControl is paving the way for faster approvals, improved patient outcomes, and the development of life-saving interventions.
25. Target e*CRF
The Target e*CRF, an exceptional EDC software, has transfigured the landscape of clinical trials, offering researchers and trial coordinators a comprehensive and user-friendly platform for streamlined data collection and study administration. With its robust features, the Target e*CRF simplifies the complex process of managing clinical trials, providing a centralized hub for efficient data tracking, analysis, and real-time collaboration.
One of the key strengths of the Target e*CRF lies in its ability to enhance patient engagement and compliance throughout the trial. By incorporating tools such as remote patient monitoring and patient-reported outcomes, the software platform facilitates data collection while ensuring participants' active involvement. Moreover, educational materials provided through the system foster participant understanding, contributing to an improved patient experience and overall satisfaction. With these features, the Target e*CRF increases the likelihood of study completion and enhances the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and the Target e*CRF prioritizes these aspects. The platform adheres to stringent privacy regulations like HIPAA and GDPR, offering robust data encryption, access controls, and comprehensive audit trails. By instilling confidence among stakeholders and ensuring data protection, the Target e*CRF enables seamless collaboration between researchers, sponsors, and regulatory bodies. This commitment to security sets the stage for efficient data management and accelerates the development of life-saving interventions, ultimately improving patient outcomes.
In addition to its patient-centric approach, the Target e*CRF boasts advanced functionalities that support researchers in conducting studies more efficiently. The software platform enables researchers to analyze larger datasets by integrating real-world data and electronic health records (EHRs). This integration facilitates post-marketing surveillance and pharmacovigilance, contributing to drug safety monitoring and regulatory compliance practices. By providing access to real-time trial data and advanced analytics tools, the Target e*CRF empowers researchers to make informed decisions promptly, accelerating the approval process and the availability of critical medical advancements.
Target e*CRF, with its EDC systems has given new direction and fresh purpose to clinical trials. Its intuitive interface, emphasis on patient engagement, and robust security measures make it an invaluable tool for researchers and trial coordinators. By streamlining the research process, promoting data accuracy, and ensuring compliance, the Target e*CRF accelerates the development of new treatments, therapies, and medical devices, ultimately leading to improved healthcare outcomes.
26. SecuTrial
SecuTrial, an untraditional EDC software, has emerged as a good hand in transforming clinical trial processes. With its comprehensive features and user-friendly interface, SecuTrial simplifies the trial process, empowering researchers and coordinators to streamline data collection, manage protocols, and automate administrative tasks. By leveraging the power of SecuTrial, the intricate web of clinical trials is untangled, paving the way for more efficient and accurate results.
One of the key challenges in clinical trials lies in engaging and retaining participants. SecuTrial addresses this challenge by incorporating tools that enhance patient engagement and compliance. Through remote patient monitoring and patient-reported outcomes, SecuTrial simplifies data collection, while educational materials foster participant understanding. By prioritizing the patient experience, SecuTrial ensures a seamless working environment for all trial components, increasing the likelihood of study completion and enhancing the reliability of trial results.
When it comes to clinical trials, data security, and compliance are paramount. SecuTrial understands the importance of safeguarding sensitive patient information and complies with HIPAA and GDPR. The software platform employs robust data encryption, access controls, and comprehensive audit trails, instilling confidence among stakeholders. This commitment to data security enables seamless collaboration between researchers, sponsors, and regulatory bodies, fostering a trusted and secure environment for conducting clinical trials.
SecuTrial streamlines the research process and accelerates the development of life-saving interventions. This software platform empowers healthcare providers to make informed treatment decisions by facilitating faster approvals and contributing to evidence-based medicine, ultimately improving patient outcomes. Additionally, SecuTrial enables the integration of real-world data and electronic health records, expanding the scope of post-marketing surveillance and pharmacovigilance. With SecuTrial, the future of clinical trials shines brighter, promising a more efficient, secure, and impactful landscape for medical research.
SecuTrial's EDC software revolutionizes clinical trial management by simplifying data collection, enhancing patient engagement, and ensuring data security. With its user-friendly interface and powerful features, SecuTrial enables researchers to conduct trials more efficiently, leading to faster approvals and the availability of life-saving interventions. By prioritizing data privacy and compliance, SecuTrial fosters collaboration among stakeholders, instilling confidence in the research process. With SecuTrial as a trusted ally, the future of clinical trials is poised for remarkable advancements in healthcare and patient outcomes.
27. Marvin
Marvin EDC software platform is a clincher in clinical trials. With its cutting-edge features and user-friendly interface, Marvin EDC simplifies the entire process, revolutionizing how researchers conduct studies and manage data. By harnessing the power of technology, Marvin EDC empowers researchers and trial coordinators to efficiently track and analyze patient data, automate administrative tasks, and streamline study protocols.
One of the standout features of Marvin EDC software is its emphasis on patient engagement and compliance. Recognizing the pivotal role of participants in generating reliable data, Marvin EDC employs a range of tools to enhance patient experience. From remote patient monitoring to patient-reported outcomes, the platform simplifies data collection while providing educational materials to foster participant understanding. By prioritizing patient engagement, Marvin EDC ensures a smooth working environment for all trial components, ultimately increasing the likelihood of study completion and enhancing the accuracy and reliability of trial results.
Undoubtedly, Security and compliance are paramount in clinical trials, and Marvin EDC leaves no stone unturned in this regard. The platform places utmost importance on data privacy and strictly adheres to industry regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, Marvin EDC instills confidence among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies. Researchers can focus on their work with peace of mind, knowing that their data is secure and compliant.
By leveraging Marvin EDC software, clinical trials can achieve new levels of efficiency and effectiveness. The platform accelerates the development of new treatments, therapies, and medical devices, contributing to advancements in evidence-based medicine. With functionalities that enable real-world data integration and electronic health record analysis, Marvin EDC facilitates post-marketing surveillance, pharmacovigilance, and drug safety monitoring. The platform's impact extends beyond clinical trials, benefiting healthcare providers by enabling informed treatment decisions and improving patient outcomes.
Marvin's EDC software platform focuses on patient engagement, data security, and compliance that sets it apart as a comprehensive and reliable solution. By simplifying the research process, empowering researchers with advanced analytics capabilities, and ensuring the highest standards of data privacy, Marvin EDC is transforming the landscape of clinical trials. Researchers and healthcare professionals can embrace this platform to unlock new possibilities, drive innovation, and improve patients' lives worldwide.
28. Data+ Research
Clinical Studio is an EDC software revolutionizing the clinical trial landscape. It provides a centralized and user-friendly platform that simplifies the complex data collection process, patient management, and study administration. With Clinical Studio, researchers and trial coordinators can seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks, all within an intuitive interface that fosters collaboration and real-time data access.
Engaging and retaining participants is vital for generating reliable data in clinical trials, and Clinical Studio excels in this area. The software offers tools to enhance patient engagement and compliance, including remote patient monitoring, simplified data collection through patient-reported outcomes, and educational materials to foster participant understanding. By prioritizing the patient experience and satisfaction, Clinical Studio ensures the smooth functioning of trial components, increasing the likelihood of study completion and improving the accuracy and reliability of trial results.
Data security and compliance are of topmost priority in clinical trials, and Clinical Studio addresses these concerns with utmost diligence. The platform prioritizes data privacy and adheres to industry regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails are implemented to safeguard sensitive patient information. This commitment to data security instills confidence among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies.
Clinical Studio's software platform is crucial in streamlining the research process and accelerating the development of new treatments, therapies, and medical devices. By enabling researchers to conduct studies more efficiently, Clinical Studio leads to faster approvals and the availability of life-saving interventions. The data collected through the platform also contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. Furthermore, Clinical Studio facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, ultimately contributing to drug safety monitoring and regulatory compliance practices.
Clinical Studio's EDC software with its comprehensive functionalities streamlines the research process, enhances data accuracy, and improves patient engagement. With its commitment to data security and compliance, Clinical Studio fosters collaboration and empowers researchers to accelerate the development of life-saving interventions. By leveraging the power of Clinical Studio, the landscape of clinical research is transformed, leading to better healthcare outcomes for patients worldwide.
29. Bioclinica Express EDC
BioClinica Express EDC software is changing clinical trial processes by providing a powerful and efficient data collection, management, and analysis platform. This innovative software simplifies the trial process, empowering researchers and coordinators to track and analyze patient data quickly. With its intuitive interface and real-time data access, Express EDC streamlines collaboration and accelerates the generation of accurate and reliable trial results.
One of the key strengths of BioClinica Express EDC software lies in its ability to enhance participant engagement and compliance. By leveraging tools for remote patient monitoring and patient-reported outcomes, the software ensures that researchers can collect data seamlessly while simplifying the experience for trial participants. Additionally, the platform provides educational materials to foster participant understanding, leading to improved patient satisfaction. Through these features, Express EDC enables researchers to optimize participant retention, ultimately increasing the likelihood of study completion.
Data security and compliance are paramount in clinical trials, and BioClinica Express EDC software prioritizes both. The platform protects sensitive patient information with robust data encryption, comprehensive access controls, and detailed audit trails. By complying with industry regulations, such as HIPAA and GDPR, Express EDC instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies. This commitment to data security safeguards patient privacy and facilitates the reliable exchange of information critical for the success of clinical trials.
BioClinica Express EDC software incorporates a suite of features that streamlines the research process, leading to faster approvals and the availability of life-saving treatments. By facilitating efficient data collection, management, and analysis, Express EDC empowers researchers to make data-driven decisions and accelerates the development of new therapies and medical devices. Furthermore, the platform's emphasis on participant engagement and data security ensures a positive trial experience for researchers and participants, ultimately contributing to improved healthcare outcomes. With BioClinica Express EDC software, the future of clinical trials is brighter than ever.
30. Triumph EDC
Triumph EDC software offers a robust and comprehensive platform that empowers researchers, trial coordinators, and participants alike. With its user-friendly interface and cutting-edge features, Triumph EDC simplifies the complex process of running clinical trials, ensuring a seamless experience from start to finish.
Triumph EDC provides a centralized hub for data collection, patient management, and study administration. Researchers and trial coordinators can track and analyze patient data, manage study protocols, and automate administrative tasks. The platform's intuitive interface promotes collaboration and enables real-time access to critical information, empowering research teams to make informed decisions easily.
One of the critical strengths of Triumph EDC lies in its focus on participant engagement and compliance. By leveraging tools that enhance patient experience, Triumph EDC simplifies data collection through patient-reported outcomes, facilitates remote patient monitoring, and offers educational materials to foster participant understanding. This commitment to participant-centricity ensures higher engagement and retention rates, generating reliable data for more accurate and meaningful trial results.
Regarding data security and compliance, Triumph EDC is second to none. The platform prioritizes data privacy and adheres to stringent regulatory standards such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, Triumph EDC instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
Triumph EDC software stands at the forefront of clinical trial management, accelerating the development of life-saving treatments, therapies, and medical devices. By streamlining the research process, researchers can conduct studies more efficiently, leading to faster approvals and improved patient outcomes. The platform's capabilities extend beyond trials, enabling the integration of real-world data and electronic health records (EHRs) for post-marketing surveillance and pharmacovigilance, contributing to enhanced drug safety monitoring and regulatory compliance practices.
In a rapidly evolving landscape, Triumph EDC empowers researchers and trial coordinators to overcome challenges and maximize the potential of their clinical trials. With its comprehensive features, user-friendly interface, and unwavering commitment to data security and participant engagement, Triumph EDC is a game-changer in clinical research, ensuring the success and impact of trials for improving healthcare outcomes.
31. Prelude Dynamics VISION
Prelude Dynamics envisions a future of clinical trials where efficiency, accuracy, and patient engagement are at the forefront. Their innovative approach combines cutting-edge technology with a deep understanding of the research process, resulting in a software platform that revolutionizes the way trials are conducted.
At the heart of Prelude Dynamics' vision is their state-of-the-art EDC software, which serves as the central hub for data collection and study administration. This intuitive platform empowers researchers and trial coordinators to seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks. By streamlining these essential components, Prelude Dynamics ensures that trials run smoothly, enabling faster approvals and the availability of life-saving interventions.
In addition to optimizing the research process, Prelude Dynamics recognizes the importance of engaging and retaining participants in clinical trials. Their EDC software incorporates tools to enhance patient engagement and compliance, such as remote patient monitoring and patient-reported outcomes. By simplifying data collection and providing educational materials, Prelude Dynamics fosters participant understanding and satisfaction. This patient-centric approach not only improves the overall trial experience but also generates reliable data for evidence-based medicine and informed treatment decisions.
Furthermore, Prelude Dynamics places a strong emphasis on data security and compliance. With the increasing importance of privacy regulations, their EDC software complies with HIPAA and GDPR standards, offering robust data encryption, access controls, and comprehensive audit trails. By prioritizing data privacy, Prelude Dynamics instills confidence among stakeholders by prioritizing data privacy, promoting seamless collaboration between researchers, sponsors, and regulatory bodies.
Prelude Dynamics with its EDC software transforms clinical trials into efficient, accurate, and patient-centered endeavors. By simplifying data collection, enhancing patient engagement, and ensuring data security, Prelude Dynamics empowers researchers to conduct studies more effectively, leading to improved healthcare outcomes and the development of life-saving interventions. With their visionary approach, Prelude Dynamics paves the way for a new era of clinical research.
32. OnCore EDC
OnCore EDC is a software solution for clinical trials specifically aimed at streamlining the entire trial process, offering researchers and coordinators a centralized hub for data collection, patient management, and study administration. By leveraging the power of this cutting-edge EDC software, clinical trials can be conducted more efficiently and effectively than ever before.
One of the key strengths of OnCore EDC lies in its ability to enhance patient engagement and compliance. Through remote patient monitoring and simplified data collection methods such as patient-reported outcomes, OnCore EDC ensures that participants stay actively involved in the trial. Furthermore, the software provides educational materials to foster participant understanding, leading to a better overall experience and higher satisfaction. By prioritizing patient engagement, OnCore EDC increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Data security and compliance are of utmost importance in clinical trials, and OnCore EDC recognizes this. The software platform adheres to strict data privacy regulations, including HIPAA and GDPR, and offers robust data encryption, access controls, and comprehensive audit trails. This commitment to data security fosters trust among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies. With OnCore EDC, researchers can focus on their work with confidence, knowing that their data is secure and compliant.
In addition to its role in streamlining the research process, OnCore EDC contributes to the development of life-saving interventions. By accelerating the approval of new treatments, therapies, and medical devices, this software platform plays a vital role in improving patient outcomes. The data collected through OnCore EDC also contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions. Furthermore, the integration of real-world data and electronic health records allows for post-marketing surveillance and pharmacovigilance, enhancing drug safety monitoring and regulatory compliance practices.
OnCore EDC has comprehensive functionalities, focus on patient engagement, and commitment to data security that sets it apart from other EDC software solutions. With OnCore EDC, researchers can conduct trials more efficiently, leading to faster approvals, improved patient care, and, ultimately, the development of life-saving interventions. This software platform is revolutionizing clinical research and paving the way for a brighter future in healthcare.
33. CRFweb
CRFweb, offers an EDC software platform, that empowers a clinical trial by revolutionizing data collection, management, and analysis. With its user-friendly interface and robust functionalities, CRFweb simplifies the complex process of conducting clinical research, empowering researchers and trial coordinators to optimize their workflows and achieve more accurate and reliable results.
At the heart of CRFweb's success is its comprehensive approach to patient engagement and compliance. Recognizing that successful trial completion hinges on engaging and retaining participants, CRFweb leverages cutting-edge tools to enhance patient involvement. The platform enables remote patient monitoring, streamlines data collection through patient-reported outcomes, and provides educational resources to foster participant understanding. By prioritizing patient experience and satisfaction, CRFweb ensures the smooth functioning of trial components, increasing the likelihood of study completion and improving the accuracy of trial results.
Data security and compliance are paramount in clinical trials, and CRFweb addresses these concerns with utmost diligence. The platform upholds strict data privacy standards, aligning with regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails fortify the platform's security measures, instilling confidence among stakeholders and fostering seamless collaboration between researchers, sponsors, and regulatory bodies. With CRFweb, researchers can focus on their crucial work, confident in the knowledge that patient data is protected with the highest level of integrity.
By streamlining the research process and accelerating the development of new treatments and therapies, CRFweb's EDC software is poised to reshape the future of clinical trials. Its efficient functionalities empower researchers to conduct studies more effectively, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through CRFweb contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. The platform's integration capabilities also facilitate the analysis of real-world data and electronic health records, expanding the scope of post-marketing surveillance and enhancing pharmacovigilance practices.
CRFweb's EDC software provides a user-friendly interface, enhancing patient engagement, prioritizing data security, and streamlining research processes, CRFweb empowers researchers to conduct studies more efficiently and generate reliable results. With its transformative capabilities, CRFweb paves the way for a future where clinical trials are conducted with greater precision, speed, and patient-centricity, ultimately advancing healthcare and improving patient outcomes.
34. Nextrials Prism
Introducing Nextrials Prism, the cutting-edge EDC software that commits to streamlining the research process and maximizing efficiency, Nextrials Prism offers a comprehensive platform for data collection, patient management, and study administration. By leveraging its intuitive interface and advanced functionalities, researchers and trial coordinators can seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks.
In the execution of clinical trials, engaging and retaining participants is paramount for generating reliable data. Nextrials Prism understands this critical need and employs tools to enhance patient engagement and compliance. By facilitating remote patient monitoring and simplifying data collection through patient-reported outcomes, this software platform ensures a smooth experience for participants. Moreover, it goes the extra mile by providing educational materials that foster participant understanding, ultimately improving the overall patient experience and satisfaction.
Security and compliance are top priorities in the realm of clinical trials, and Nextrials Prism takes them seriously. With a dedication to data privacy, Nextrials Prism adheres to stringent regulations such as HIPAA and GDPR. Through robust data encryption, access controls, and comprehensive audit trails, the platform offers a secure environment for sensitive patient data. This commitment to data security instills confidence among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies.
Nextrials Prism goes beyond simply streamlining the research process—it accelerates the development of new treatments, therapies, and medical devices. By enabling researchers to conduct studies more efficiently, it leads to faster approvals and the availability of life-saving interventions. Additionally, the data collected through Nextrials Prism contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. The platform also facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, ultimately enhancing drug safety monitoring and regulatory compliance practices.
With Nextrials Prism, clinical trials reach new levels of efficiency, accuracy, and reliability. By harnessing the power of this state-of-the-art EDC software, researchers can confidently navigate the complex landscape of clinical research, delivering impactful results that shape the future of healthcare.
35. eClinicalOS
eClinicalOS is a powerful EDC software with its comprehensive features and user-friendly interface. eClinicalOS simplifies the entire research process, enabling researchers and trial coordinators to collect, manage, and analyze critical patient data efficiently. This cloud-based platform is a centralized hub for study administration and patient management, ensuring seamless collaboration and real-time data access.
One of the key advantages of eClinicalOS is its focus on enhancing patient engagement and compliance. Engaging and retaining participants in clinical trials is crucial for generating reliable data; eClinicalOS recognizes this. Through tools such as remote patient monitoring and patient-reported outcomes, the software facilitates data collection while providing educational materials to foster participant understanding. By prioritizing patient experience and satisfaction, eClinicalOS improves the accuracy and reliability of trial results, increasing the likelihood of study completion.
Security and compliance are paramount in clinical trials, considering the sensitive nature of patient data. eClinicalOS addresses this concern by prioritizing data privacy and complying with regulations like HIPAA and GDPR. The platform ensures robust data encryption, access controls, and comprehensive audit trails, instilling confidence among stakeholders and enabling seamless collaboration between researchers, sponsors, and regulatory bodies. This commitment to data security ensures that patient confidentiality is maintained throughout the research process.
eClinicalOS EDC software streamlines the research process and accelerates the development of new treatments and therapies. By providing efficient data management, real-time analytics, and integration with electronic health records, eClinicalOS empowers researchers to conduct studies more efficiently and make informed treatment decisions. The platform's dedication to patient engagement and data security further reinforces its value in clinical trials, ultimately leading to improved patient outcomes and advancing evidence-based medicine.
36. OnQ RDE
In the fast-paced world of clinical trials, researchers and trial coordinators require a solution that streamlines the entire process while ensuring accuracy, security, and efficiency. OnQ RDE is an innovative software platform that has revolutionized the clinical research. With its robust Electronic Data Capture (EDC) software, OnQ RDE provides an all-encompassing solution for running clinical trials.
OnQ RDE's EDC software serves as the backbone of the platform, offering a centralized hub for data collection, management, and analysis. Researchers and trial coordinators can now seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks. The intuitive interface of OnQ RDE allows for real-time collaboration and easy access to critical data, empowering researchers to make informed decisions quickly.
One of the key strengths of OnQ RDE lies in its focus on patient engagement and compliance. Leveraging the power of EDC software, OnQ RDE facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to enhance participant understanding. By prioritizing the patient experience and satisfaction, OnQ RDE ensures higher participant retention rates, leading to more reliable and accurate trial results.
Security and compliance are paramount when dealing with sensitive patient data, and OnQ RDE recognizes this critical need. The platform implements robust data encryption, access controls, and comprehensive audit trails, ensuring data privacy and compliance with industry regulations such as HIPAA and GDPR. This commitment to data security builds trust among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
OnQ RDE has transformed the landscape of clinical trials with its state-of-the-art EDC software. By streamlining the research process and prioritizing patient engagement, OnQ RDE empowers researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, the platform's commitment to data security and compliance ensures the integrity and privacy of patient information. With OnQ RDE, the future of clinical trials is brighter than ever, enabling researchers to drive medical advancements and improve patient outcomes.
37. ACI Clinical
ACI Clinical, a leading provider in the clinical trial industry, has introduced their cutting-edge EDC software, to help researchers conduct studies and gather critical data. This comprehensive platform offers a centralized hub for data collection, patient management, and study administration, simplifying the entire clinical trial process. With ACI Clinical's EDC software, researchers and trial coordinators can seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks, leading to more efficient and accurate clinical trials.
One of the key advantages of ACI Clinical's EDC software is its focus on enhancing patient engagement and compliance. By leveraging tools such as remote patient monitoring and patient-reported outcomes, the software facilitates data collection while ensuring participants remain actively involved in the trial. Additionally, ACI Clinical provides educational materials to promote participant understanding, ultimately improving patient experience and satisfaction. By prioritizing patient engagement, ACI Clinical's EDC software increases the likelihood of study completion, generating reliable data for informed decision-making.
Data security and compliance are paramount in clinical trials, and ACI Clinical's EDC software addresses these concerns effectively. The platform adheres to strict privacy regulations, including HIPAA and GDPR, ensuring the protection of sensitive patient data. Robust data encryption, access controls, and comprehensive audit trails contribute to maintaining the integrity and confidentiality of trial information. ACI Clinical's commitment to data security fosters trust and collaboration among stakeholders, enabling researchers, sponsors, and regulatory bodies to work seamlessly together.
ACI Clinical's EDC software contributes aggressively in accelerating the development of new treatments, therapies, and medical devices. By streamlining the research process, the platform empowers researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through ACI Clinical's EDC software contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. Additionally, the platform facilitates the integration of real-world data and electronic health records, enhancing post-marketing surveillance and pharmacovigilance practices, ultimately contributing to drug safety monitoring and regulatory compliance.
ACI Clinical's EDC software is a powerful tool that helps conduct clinical trial methodically and efficiently. With its streamlined data collection, enhanced patient engagement, and robust data security features, the platform empowers researchers to conduct more efficient trials, leading to improved healthcare outcomes. ACI Clinical's commitment to innovation and data integrity ensures that the future of clinical trials is marked by precision, reliability, and accelerated progress in medical research.
38. Quanticate EDC
Quanticate EDC software with its comprehensive and cutting-edge platform is designed to optimize data collection, analysis, and study administration, Quanticate EDC empowers researchers and trial coordinators to navigate the complexities of clinical trials with ease and efficiency.
At the core of Quanticate EDC software is its centralized hub, which serves as a command center for seamless collaboration and real-time data access. This intuitive interface enables researchers to track and analyze patient data, manage study protocols, and automate administrative tasks. Quanticate EDC eliminates the need for fragmented systems and enables a holistic approach to trial management by providing a single platform for these essential functions.
One of the key strengths of Quanticate EDC software lies in its commitment to enhancing patient engagement and compliance. Recognizing that participant involvement is crucial for generating reliable data, Quanticate EDC employs tools that foster a positive patient experience. Quanticate EDC ensures that participants are actively involved in the trial process, from remote patient monitoring to simplified data collection through patient-reported outcomes. Quanticate EDC empowers patients to play an active role in their healthcare journey by providing educational materials and supporting participant understanding.
Security and compliance are paramount in clinical trials, and Quanticate EDC software prioritizes data privacy and protection. With robust data encryption, access controls, and comprehensive audit trails, Quanticate EDC ensures that sensitive patient data remains secure. This commitment to data security instills confidence among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies.
Quanticate EDC software is a dominant development in the field of clinical trial management. By streamlining the research process, accelerating the development of new treatments, and improving patient outcomes, Quanticate EDC empowers researchers to conduct studies more efficiently. With its emphasis on data accuracy, patient engagement, and compliance, Quanticate EDC is a powerful tool that brings innovation and effectiveness to clinical trials.
39. Forte EDC
Forte EDC software is a technological tool for empowering researchers and streamlining the entire research process. With its comprehensive suite of features, Forte EDC enables efficient data collection, management, and analysis, resulting in accelerated trial completion and robust results.
At the core of Forte EDC lies its user-friendly interface, providing researchers with an intuitive platform for data collection and patient management. By centralizing data in one location, researchers can easily access and analyze real-time information, facilitating seamless collaboration among study teams. The software's sophisticated tools automate administrative tasks, allowing researchers to focus on the essential aspects of their trials. Forte EDC empowers investigators to track study protocols effectively, ensuring adherence and enhancing the accuracy of data.
Patient engagement and compliance are pivotal to successful clinical trials, and Forte EDC addresses these challenges with innovative solutions. The software integrates patient-reported outcomes, simplifying data collection and minimizing the burden on participants. By providing educational resources and facilitating remote patient monitoring, Forte EDC fosters a positive experience for patients throughout their involvement in the trial. This emphasis on patient satisfaction and engagement contributes to higher retention rates and more reliable data.
Data security is a paramount concern in clinical trials, and Forte EDC prioritizes the protection of sensitive patient information. The software adheres to stringent security protocols and compliance standards, such as HIPAA and GDPR, ensuring data privacy and confidentiality. Forte EDC employs robust encryption techniques, access controls, and comprehensive audit trails to instill confidence among stakeholders, including researchers, sponsors, and regulatory bodies.
Forte EDC software is a decisive resource in the realm of clinical trials. By simplifying data collection, automating administrative tasks, and prioritizing patient engagement, it facilitates the efficient completion of trials. With its emphasis on data security and compliance, Forte EDC provides researchers with a reliable and secure platform for managing their studies. By leveraging the power of Forte EDC, researchers can accelerate the development of life-saving interventions and contribute to evidence-based medicine, ultimately improving patient outcomes and advancing healthcare as a whole.
40. Flex Databases EDC
Flex Databases EDC software offers a powerful and comprehensive solution for researchers and trial coordinators. With its advanced features and user-friendly interface, Flex Databases EDC streamlines the entire trial process, from data collection to analysis, resulting in increased efficiency and reliable results.
One of the key strengths of Flex Databases EDC software lies in its ability to engage and retain participants in clinical trials. By leveraging innovative patient engagement and compliance tools, this platform ensures that patients remain actively involved throughout the trial. Remote patient monitoring, simplified data collection through patient-reported outcomes, and educational materials are just some features that enhance patient experience and satisfaction. By prioritizing participant engagement, Flex Databases EDC software enables researchers to gather high-quality data, improving the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and Flex Databases EDC software understands the importance of protecting sensitive patient information. With robust data encryption, comprehensive access controls, and detailed audit trails, this platform ensures data privacy and complies with strict regulations such as HIPAA and GDPR. Flex Databases EDC software facilitates seamless collaboration and promotes trust throughout the trial process by instilling confidence in stakeholders, including researchers, sponsors, and regulatory bodies.
Moreover, Flex Databases EDC software accelerates the development of new treatments, therapies, and medical devices. By streamlining research processes and providing efficient functionalities, this platform enables researchers to conduct studies more effectively, leading to faster approvals and the availability of life-saving interventions. Additionally, the data collected through Flex Databases EDC software contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. By facilitating the integration of real-world data and electronic health records, this platform supports post-marketing surveillance and pharmacovigilance, enhancing drug safety monitoring and regulatory compliance practices.
Summing up, Flex Databases EDC software remodels the management of clinical trials, offering a comprehensive and efficient solution for researchers and trial coordinators. Its focus on participant engagement, data security, and streamlined research processes empowers researchers to conduct studies effectively and generate reliable results. With its wide range of functionalities and commitment to data privacy, Flex Databases EDC software plays a crucial role in advancing healthcare and improving patient outcomes.
41. DataLabs EDC
DataLabs EDC software is transforming the way research is conducted and accelerating the development of new treatments and therapies. With its comprehensive suite of tools and features, DataLabs EDC simplifies the entire trial process, from data collection to study administration, enabling researchers to streamline their workflow and achieve more accurate and reliable results.
One of the key strengths of DataLabs EDC is its ability to enhance patient engagement and compliance. By leveraging innovative techniques and technologies, the software platform facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to ensure participants' understanding. This focus on improving the patient experience not only increases the likelihood of study completion but also enhances the accuracy and reliability of trial data.
Data security and compliance are of utmost importance in clinical trials, and DataLabs EDC software excels in this aspect. The platform prioritizes data privacy and meets the stringent requirements of regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure that sensitive patient information remains secure. This commitment to data security instills confidence among stakeholders and fosters seamless collaboration between researchers, sponsors, and regulatory bodies.
By leveraging DataLabs EDC software, researchers can expedite the trial process and bring life-saving interventions to market more swiftly. The platform's advanced functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and improved patient outcomes. Additionally, the data collected through DataLabs EDC contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and further enhance patient care.
DataLabs EDC software represents a significant advancement in the field of clinical trials. With its comprehensive suite of features, focus on patient engagement, and commitment to data security, it empowers researchers to conduct studies more efficiently and effectively. By embracing this cutting-edge technology, researchers can unlock new insights, accelerate medical advancements, and ultimately improve the lives of patients around the world.
42. Nextrials NexEDC
Nextrials NexEDC focuses on efficiency, accuracy, and participant engagement. It is empowering researchers and trial coordinators to streamline the trial process and generate reliable data.
The NexEDC platform offers a comprehensive suite of features for data collection, patient management, and study administration. By centralizing a featuresll trial-related activities, researchers can easily track and analyze patient data, manage study protocols, and automate administrative tasks. The user-friendly interface promotes seamless collaboration and real-time data access, enabling researchers to make informed decisions promptly.
One of the key strengths of NexEDC lies in its tools for enhancing participant engagement and compliance. The platform facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By prioritizing patient experience and satisfaction, NexEDC ensures a smooth workflow for trial components, increasing the likelihood of study completion and improving the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and NexEDC takes these concerns seriously. The platform adheres to the highest standards of data privacy and complies with regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure the integrity and confidentiality of sensitive patient information. This commitment to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
NexEDC's powerful EDC software accelerates the development of new treatments, therapies, and medical devices, and contributes to evidence-based medicine. By enabling efficient study conduct, faster approvals, and the availability of life-saving interventions, NexEDC plays a crucial role in advancing healthcare outcomes. Additionally, the platform facilitates the integration of real-world data and electronic health records, enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance. NexEDC is shaping the future of clinical trials, revolutionizing the way research is conducted and bringing us closer to breakthrough treatments and improved patient care.
43. TrialOne
TrialOne platform transforms the management of clinical trials through its state-of-the-art Electronic Data Capture (EDC) software. With its comprehensive suite of features and intuitive interface, TrialOne simplifies the entire clinical trial process, empowering researchers and trial coordinators to manage and analyze patient data while automating administrative tasks efficiently. By revolutionizing data collection, TrialOne is remodelling how we conduct clinical trials.
One of the key strengths of TrialOne lies in its ability to enhance patient engagement and compliance. Recognizing that participant involvement is critical to generating reliable data, TrialOne offers tools for remote patient monitoring, seamless data collection through patient-reported outcomes, and educational materials that foster participant understanding. By prioritizing the patient experience and satisfaction, TrialOne ensures that clinical trials are conducted smoothly, increasing the likelihood of study completion and improving the accuracy and reliability of trial results.
Data security is a paramount concern in clinical trials, and TrialOne has taken proactive measures to address this issue. The platform adheres to strict privacy regulations such as HIPAA and GDPR, providing robust data encryption, access controls, and comprehensive audit trails. This commitment to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies. With TrialOne, researchers can focus on their work, knowing that patient data is protected with the highest level of security.
Moreover, TrialOne is not just a tool for conducting clinical trials but a catalyst for medical advancements. By streamlining the research process and accelerating the development of new treatments, therapies, and medical devices, TrialOne empowers researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. The data collected through TrialOne contributes to evidence-based medicine, allowing healthcare providers to make informed treatment decisions and improve patient outcomes. Furthermore, TrialOne facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, greatly enhancing drug safety monitoring and regulatory compliance practices.
TrialOne provides advanced EDC software with focus on patient engagement, data security, and medical advancements. It offers researchers and trial coordinators an unparalleled platform to conduct studies efficiently, accurately, and confidently. By harnessing the power of TrialOne, we can accelerate the pace of medical discoveries and ultimately improve the lives of countless individuals worldwide.
44. Clinpal
Clinpal, an innovative platform for running clinical trials, transforms the medical research landscape through its advanced electronic data capture (EDC) software. With its comprehensive suite of tools and streamlined processes, Clinpal simplifies trial management, enhances patient engagement, and ensures data security, all while accelerating the development of life-saving interventions.
At the core of Clinpal's success is its EDC software, which offers a centralized hub for data collection, patient management, and study administration hub. Researchers and trial coordinators can seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks through the platform's intuitive interface. This efficiency level enables faster approvals, shorter trial durations, and more reliable results.
One of Clinpal's standout features is its focus on patient engagement and compliance. The platform enhances data collection by utilizing remote patient monitoring and patient-reported outcomes while simplifying the participant experience. Additionally, Clinpal provides educational materials to foster participant understanding, ensuring their active involvement throughout the trial. This emphasis on patient satisfaction not only improves the accuracy and reliability of trial results but also increases the likelihood of study completion.
Data security and compliance are paramount in clinical trials, and Clinpal excels in them. The platform adheres to strict privacy regulations such as HIPAA and GDPR, prioritizing data encryption, access controls, and comprehensive audit trails. This commitment to data security fosters trust among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies. Clinpal's dedication to maintaining the privacy and integrity of sensitive patient data is a cornerstone of its success.
Clinpal's EDC software streamlines the research process, accelerating treatment development, and ensuring data accuracy and security. Its advanced functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. By prioritizing patient engagement and compliance, Clinpal enhances the overall trial experience, empowering participants to contribute meaningfully to scientific advancements. With Clinpal, clinical research can be more meticulously handled.
45. eClinicalPlus
eClinicalPlus provides a comprehensive and innovative electronic data capture (EDC) software. This cutting-edge platform simplifies the clinical trial process, offering researchers and trial coordinators a centralized hub for data management, patient engagement, and study administration. With its intuitive interface and robust functionalities, eClinicalPlus is poised to revolutionize clinical trials.
At the heart of eClinicalPlus lies its powerful EDC software, which enables researchers to capture, record, and analyze patient data electronically. Gone are the days of cumbersome paper-based processes, as eClinicalPlus automates data collection, eliminating errors and improving data accuracy. This saves valuable time and resources and enhances collaboration among research teams by centralizing data in a secure and standardized format. By leveraging eClinicalPlus' advanced analytics tools, researchers can gain real-time access to trial data, monitor study progress, and make informed decisions promptly. The platform's data-driven insights and comprehensive reporting capabilities empower researchers to identify trends and extract valuable insights effortlessly, ultimately leading to more efficient and effective clinical trials.
One of the key strengths of eClinicalPlus lies in its commitment to patient engagement and satisfaction. The platform incorporates tools and features to enhance participant experience throughout the trial. With remote patient monitoring, patient-reported outcomes, and educational materials, eClinicalPlus ensures that participants remain engaged and compliant. By creating a user-friendly interface accessible from mobile devices and supporting multilingual interactions, eClinicalPlus makes it easier for patients to contribute their data and actively participate in research studies. This emphasis on patient-centricity not only broadens the diversity of the participant pool but also improves the generalizability of trial results, enabling better-informed healthcare decisions and more effective treatments.
Security and compliance are of paramount importance in the realm of clinical trials, and eClinicalPlus recognizes this imperative. The platform prioritizes data privacy and adheres to the highest security standards, including compliance with HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure the confidentiality and integrity of sensitive patient information. By fostering trust among stakeholders, including researchers, sponsors, and regulatory bodies, eClinicalPlus facilitates seamless collaboration and accelerates the pace of clinical research.
eClinicalPlus contributes innovatively in transforming the landscape of clinical trials with its advanced EDC software. By providing a comprehensive platform for data management, patient engagement, and study administration, eClinicalPlus simplifies and accelerates the research process. Through its streamlined data capture, advanced analytics, and commitment to patient-centricity, eClinicalPlus empowers researchers to conduct more efficient and effective trials, ultimately improving healthcare outcomes and driving the development of life-saving interventions.
46. RENO EDC
The Reno EDC software for running clinical trials offers a comprehensive and cloud-based platform that simplifies the entire clinical trial process, revolutionizing how data is collected, managed, and analyzed. With a centralized hub for data collection, patient management, and study administration, researchers and trial coordinators can now track and analyze patient data more efficiently than ever.
One of the key features of the Reno EDC software is its focus on enhancing patient engagement and compliance. By utilizing tools for remote patient monitoring, simplifying data collection through patient-reported outcomes, and providing educational materials, the software ensures that participants are actively involved and committed to the trial. This improves the patient experience, increases the likelihood of study completion, and enhances the accuracy and reliability of trial results.
Data security and compliance are of utmost importance in clinical trials, and the Reno EDC software understands this crucial aspect. With robust data encryption, access controls, and comprehensive audit trails, the software prioritizes data privacy and complies with HIPAA and GDPR. This commitment to data security instills confidence among stakeholders and enables seamless collaboration between researchers, sponsors, and regulatory bodies.
Overall, the Reno EDC software is a powerful tool that streamlines the research process and accelerates the development of new treatments, therapies, and medical devices. Its functionalities help researchers conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions, and it also contributes to evidence-based medicine. By integrating real-world data and electronic health records (EHRs), the software enables the analysis of larger datasets, further enhancing drug safety monitoring and regulatory compliance practices.
The Reno EDC software is a pathsetting solution that transforms the landscape of clinical trials. By simplifying data collection, ensuring patient engagement, and prioritizing data security, this software empowers researchers to conduct more efficient and effective trials, ultimately improving healthcare outcomes for patients worldwide.
47. Anju ClinPlus EDC
Anju ClinPlus EDC has a comprehensive suite of features and user-friendly interface, which sets a new standard for efficiency, accuracy, and collaboration in the research community. Let's explore how this innovative EDC software is transforming the way clinical trials are conducted.
ClinPlus EDC is designed to streamline the entire clinical trial process, from data collection to analysis. This software enables seamless tracking and management of patient data, study protocols, and administrative tasks by offering a centralized platform for researchers and trial coordinators. Its intuitive interface promotes real-time collaboration, empowering teams to work together effortlessly by offering a centralized platform for researchers and trial coordinators.
One of the key strengths of ClinPlus EDC lies in its commitment to patient engagement and compliance. The software provides tools to enhance participant involvement, simplifying data collection through patient-reported outcomes and enabling remote patient monitoring. By fostering a positive patient experience, ClinPlus EDC ensures higher engagement and retention rates, leading to more reliable and comprehensive data sets.
Data security and compliance are of paramount importance in clinical trials, and ClinPlus EDC excels in this area. The software prioritizes the privacy and protection of sensitive patient information, adhering to stringent regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, ClinPlus EDC instills confidence among stakeholders and facilitates smooth collaboration between researchers, sponsors, and regulatory bodies.
Anju ClinPlus EDC in the realm of clinical trials streamlines the research process and optimizes data management. It accelerates the development of life-saving interventions, therapies, and medical devices. The software's advanced functionalities empower researchers to conduct studies more efficiently, leading to faster approvals and improved patient outcomes. With its focus on patient engagement, data security, and compliance, ClinPlus EDC sets a new standard for excellence in clinical research, ensuring the success and reliability of every trial it supports.
48. Target e*CTR
Target e*CTR for running clinical trials plays a pivotal role in ensuring the success of these crucial research endeavors. One software platform that excels in this domain is Clinical Studio, a comprehensive cloud-based solution that simplifies the entire clinical trial process. With its intuitive interface and powerful tools, Clinical Studio enhances patient engagement, improves data accuracy, and ensures the secure management of sensitive information.
Clinical Studio's commitment to engaging and retaining participants is evident through its innovative features. The platform facilitates remote patient monitoring and simplifies data collection using patient-reported outcomes. Providing educational materials, it fosters participant understanding and compliance. These capabilities enhance the overall patient experience and satisfaction, ultimately increasing the likelihood of study completion and generating reliable data.
Data security and compliance are paramount in clinical trials, and Clinical Studio addresses these concerns effectively. The platform prioritizes data privacy and adheres to strict regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, Clinical Studio instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
By streamlining the research process and accelerating the development of new treatments, therapies, and medical devices, Clinical Studio contributes to the advancement of healthcare. Its functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, the data collected through Clinical Studio facilitates evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. The platform integrates real-world data and electronic health records (EHRs), enabling post-marketing surveillance and pharmacovigilance, contributing to drug safety monitoring and regulatory compliance practices.
Clinical Studio has a robust EDC software platform for revamping clinical trial management. Through its patient engagement features, data security measures, and contributions to evidence-based medicine, Clinical Studio drives the success and effectiveness of clinical research. Its dedication to simplifying processes and improving patient outcomes is a valuable tool for researchers and healthcare professionals.
49. Formedix Origin
Formedix Origin offers a powerful EDC software platform. Designed to simplify and optimize the process, Origin empowers researchers and trial coordinators to run trials more efficiently and precisely. With its intuitive interface and comprehensive capabilities, Origin enables seamless collaboration, real-time data access, and enhanced patient engagement.
At the heart of Origin is its robust data collection and management system. Researchers can effortlessly track and analyze patient data, ensuring the accuracy and reliability of trial results. The platform automates administrative tasks and provides tools for remote patient monitoring and patient-reported outcomes, simplifying data collection and fostering participant compliance. By prioritizing patient experience and satisfaction, Origin increases the likelihood of study completion, generating more reliable data.
Data security and compliance are paramount in clinical trials, and Formedix Origin addresses these concerns head-on. The platform adheres to strict data privacy regulations such as HIPAA and GDPR. It incorporates robust data encryption, access controls, and comprehensive audit trails, instilling confidence among stakeholders. Researchers, sponsors, and regulatory bodies can collaborate seamlessly, knowing patient data is protected.
Formedix Origin goes beyond simplifying clinical trials. By streamlining the research process, it accelerates the development of life-saving interventions. The platform enables faster approvals and contributes to evidence-based medicine. Integrating electronic health records (EHRs) and real-world data allows for post-marketing surveillance and pharmacovigilance, enhancing drug safety monitoring and regulatory compliance practices. Origin's impact extends beyond the confines of clinical trials, positively influencing healthcare services and patient outcomes.
Formedix Origin is reshaping the landscape of clinical trials with its innovative EDC software platform. By optimizing data collection, enhancing collaboration, and ensuring data security, Origin empowers researchers to conduct trials more efficiently and effectively. With its patient-centric approach and commitment to advancing healthcare, Origin drives meaningful change in the industry, ultimately leading to improved patient care and better treatment outcomes.
50. Medical Data Solutions (MDSol)
MDSol, the leading provider of Medical Data Solutions, offers groundbreaking EDC software that nudges clinical trials towards ace management. With a relentless focus on efficiency, accuracy, and patient engagement, MDSol's platform is a game-changer in medical research.
At the core of MDSol's offering is its comprehensive EDC software, which serves as a centralized data collection, management, and analysis hub. Researchers and trial coordinators can seamlessly track and analyze patient data, manage study protocols, and automate administrative tasks. This intuitive platform simplifies the research process and fosters collaboration and real-time data access, enabling teams to work together seamlessly regardless of location.
One of the key strengths of MDSol's EDC software lies in its ability to enhance patient engagement and compliance. By leveraging tools such as remote patient monitoring and patient-reported outcomes, MDSol ensures that participants remain actively involved in the trial process. Additionally, the platform provides educational materials to promote participant understanding, further improving the overall patient experience. Through these initiatives, MDSol increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and MDSol understands this critical aspect. Their EDC software prioritizes data privacy and complies with industry regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, MDSol's commitment to data security instills confidence among stakeholders, fostering collaboration between researchers, sponsors, and regulatory bodies.
MDSol's EDC software is a transformative tool that accelerates the development of new treatments, therapies, and medical devices. By streamlining the research process, improving patient engagement, and ensuring data security, MDSol empowers researchers to conduct studies more efficiently. The platform's impact extends beyond clinical trials, contributing to evidence-based medicine, post-marketing surveillance, and pharmacovigilance.
51. Flex Databases ClinOps Suite
Flex Databases ClinOps Suite offers many functionalities that streamline the entire trial process, from data collection to study administration. With ClinOps Suite, researchers and trial coordinators access a centralized hub for efficient data tracking, analysis, and management, all within a user-friendly and intuitive interface.
One of the key strengths of ClinOps Suite lies in its focus on patient engagement and compliance. By leveraging innovative tools, such as remote patient monitoring and patient-reported outcomes, the software enables researchers to enhance participant engagement and ensure high levels of compliance throughout the trial. Additionally, the platform provides educational materials to foster participant understanding, further improving the overall patientcollaborateBy prioritizing participant satisfaction, ClinOps Suite increases the likelihood of study completion and enhances the accuracy and reliability of trial results.
Data security is of paramount importance in clinical trials, and ClinOps Suite excels in this area. The platform is designed with robust data encryption, access controls, and comprehensive audit trails to ensure the privacy and compliance of sensitive patient data. With its adherence to HIPAA and GDPR regulations, ClinOps Suite instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies. This commitment to data security is vital in an era where privacy breaches can have significant consequences.
Flex Databases ClinOps Suite can be safely included amongst tipping point EDC software systems in clinical trial management. Its powerful EDC software empowers researchers and trial coordinators to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. The platform's focus on patient engagement, along with its robust data security measures, ensures a seamless and reliable trial process. By leveraging ClinOps Suite, researchers contribute to evidence-based medicine, improve patient outcomes, and accelerate the development of new treatments, therapies, and medical devices.
52. DSG eCaseLink EDC
DSG eCaseLink EDC offers a robust and efficient platform for researchers and trial coordinators. With its comprehensive features and user-friendly interface, eCaseLink simplifies data collection, patient management, and study administration, making the entire process seamless and streamlined.
Engaging and retaining participants is crucial for generating reliable data, and eCaseLink excels in this aspect. The software provides tools for enhancing patient engagement and compliance, such as remote patient monitoring and patient-reported outcomes. Moreover, eCaseLink offers educational materials that foster participant understanding, ensuring a positive and informed experience throughout the trial. By prioritizing patient satisfaction and experience, eCaseLink maximizes the likelihood of study completion and enhances the accuracy and reliability of trial results.
Security and compliance are of paramount importance when dealing with sensitive patient data, and eCaseLink understands this. The software platform adheres to stringent privacy standards, including compliance with HIPAA and GDPR regulations. Robust data encryption, access controls, and comprehensive audit trails ensure the confidentiality and integrity of patient information. This commitment to data security instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
The impact of eCaseLink EDC software extends beyond the completion of clinical trials. By streamlining the research process, it accelerates the development of new treatments, therapies, and medical devices. Researchers can conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Additionally, the data collected through eCaseLink contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. The software platform also facilitates the integration of real-world data and electronic health records, enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, ultimately enhancing drug safety monitoring and regulatory compliance practices.
DSG eCaseLink EDC software empowers researchers and trial coordinators with a comprehensive platform that simplifies the clinical trial process. Through its patient engagement, data security, and compliance features, eCaseLink ensures a positive participant experience while safeguarding sensitive information. By streamlining the research process, eCaseLink accelerates the development of life-saving interventions and contributes to evidence-based medicine. With its transformative capabilities, eCaseLink transforms the field of clinical trials and drive improvements in patient care and outcomes.
53. Fusion eClinical Suite
The Fusion eClinical Suite is an EDC platform designed to transform clinical trials. This robust EDC software has a comprehensive suite of features that streamline the entire clinical trial process, from data collection to study management. With Fusion, researchers and trial coordinators can efficiently track and analyze patient data, manage study protocols, and automate administrative tasks, all within a centralized hub.
One of the key strengths of the Fusion eClinical Suite lies in its commitment to enhancing patient engagement and compliance. The platform incorporates innovative tools for remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By prioritizing the patient experience and satisfaction, Fusion ensures a smooth workflow of trial components, increasing the likelihood of study completion and improving the accuracy and reliability of trial results.
Security and compliance are paramount in clinical trials, and the Fusion eClinical Suite excels. The platform prioritizes data privacy and adheres to strict regulatory standards such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails provide a secure environment for sensitive patient data. With Fusion, stakeholders can have confidence in the platform's commitment to data security, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
In addition to facilitating individual clinical trials, the Fusion eClinical Suite contributes to advancing medical research. By streamlining the research process and accelerating the development of new treatments, therapies, and medical devices, Fusion plays a crucial role in saving lives and improving patient outcomes. The platform's capabilities also extend beyond individual trials, allowing for integrating real-world data and electronic health records (EHRs) for post-marketing surveillance and pharmacovigilance. This integration contributes to drug safety monitoring and regulatory compliance practices, making the Fusion eClinical Suite an indispensable tool for researchers and healthcare providers.
The Fusion eClinical Suite gives a faithful commitment to patient engagement, data security, and research advancement. By leveraging the power of this cutting-edge EDC software, researchers can conduct trials more efficiently, leading to faster approvals and the availability of life-saving interventions. The Fusion eClinical Suite sets a new standard for clinical trial management, making it an invaluable asset for researchers, sponsors, and healthcare professionals worldwide.
54. Siron Clinical
Siron Clinical, is a cutting-edge EDC software platform for seamless integration of data collection, patient management, and study administration. This powerful cloud-based solution empowers researchers and trial coordinators to efficiently track and analyze patient data, automate administrative tasks, and manage study protocols. With its intuitive interface and real-time data access, Siron Clinical ensures streamlined collaboration and a smooth trial process.
One of the key challenges in clinical trials is engaging and retaining participants to generate reliable data. Siron Clinical addresses this by incorporating tools for enhancing patient engagement and compliance. The platform facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By prioritizing patient experience and satisfaction, Siron Clinical creates an environment encouraging study completion, resulting in accurate and reliable trial results.
Security and compliance are paramount when handling sensitive patient data in clinical trials, and Siron Clinical takes this responsibility seriously. The platform prioritizes data privacy and adheres to stringent regulations such as HIPAA and GDPR. With robust data encryption, access controls, and comprehensive audit trails, Siron Clinical instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies. By ensuring the security of patient data, Siron Clinical provides a foundation of trust that allows researchers to focus on advancing medical treatments and improving patient outcomes.
The impact of Siron Clinical extends beyond individual trials. By streamlining the research process and accelerating the development of new treatments, therapies, and medical devices, the platform contributes to advancing evidence-based medicine. It enables researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, Siron Clinical facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance. This contributes to drug safety monitoring and regulatory compliance practices, ensuring the continued improvement of healthcare services.
Siron Clinical is a powerful EDC software platform that transforms the clinical trial landscape. With its comprehensive features for data collection, patient management, and study administration features, Siron Clinical simplifies the research process and enhances the accuracy and reliability of trial results. By prioritizing patient engagement, ensuring data security and compliance, and facilitating the integration of real-world data, Siron Clinical paves the way for more efficient trials and improved healthcare outcomes. Researchers can leverage the capabilities of Siron Clinical to accelerate medical advancements and provide better treatments for patients in need.
55. CareLex EDC
CareLex EDC software empowers researchers and healthcare providers with an innovative platform that simplifies data collection, analysis, and management. With its comprehensive suite of features, CareLex EDC streamlines the research process, enhances patient engagement, and ensures data security and compliance, setting a new standard in the industry.
At the heart of CareLex EDC is its ability to efficiently capture and record patient data electronically, replacing antiquated paper-based methods. This transformation not only saves time and resources but also significantly reduces the likelihood of errors, ultimately improving data accuracy. By centralizing data in a secure and standardized format, researchers can seamlessly manage and analyze trial data, fostering collaboration and enabling real-time access to critical information.
CareLex EDC goes beyond data management by prioritizing patient engagement. Through user-friendly interfaces, mobile accessibility, and multilingual support, the software facilitates seamless participation, empowering patients to contribute their valuable data to research studies. By enhancing the patient experience and satisfaction, CareLex EDC fosters a diverse participant pool and improves the generalizability of trial results, leading to better-informed healthcare decisions and more effective treatments.
Data security is a paramount concern in clinical trials, and CareLex EDC addresses this with utmost diligence. The software adheres to rigorous security protocols, ensuring data privacy and compliance with industry standards such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails instill confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
CareLex EDC software emerges as a wholesome facilitator of clinical trials. By providing a powerful platform that streamlines data collection and analysis, enhances patient engagement, and ensures data security and compliance, CareLex EDC empowers researchers to conduct more efficient and effective trials. This innovative software accelerates the development of new treatments, therapies, and medical devices, ultimately leading to improved healthcare outcomes and the advancement of evidence-based medicine. CareLex EDC sets a new standard for excellence in clinical research, revolutionizing how trials are conducted and transforming the future of healthcare.
56. NovoClinical
NovoClinical is a cutting-edge EDC software with its comprehensive suite of features and user-friendly interface. NovoClinical empowers researchers and trial coordinators to streamline their processes and unlock new levels of efficiency. This innovative platform serves as a centralized hub for data collection, patient management, and study administration, allowing seamless collaboration and real-time access to critical information.
One of the standout features of NovoClinical is its commitment to enhancing patient engagement and compliance. Recognizing that successful trial completion hinges on effectively engaging and retaining participants, NovoClinical offers tools such as remote patient monitoring and patient-reported outcomes. By simplifying data collection and providing educational materials, the platform ensures participants a smooth and satisfying experience, ultimately leading to more reliable and accurate trial results.
Data security and compliance are paramount in clinical trials, and NovoClinical is built with these considerations in mind. The platform prioritizes data privacy and complies with strict regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails give stakeholders the confidence to collaborate effectively while safeguarding sensitive patient information. This commitment to security fosters trust between researchers, sponsors, and regulatory bodies, enabling a more efficient and reliable clinical trial process.
NovoClinical's impact extends beyond the realm of individual trials. The platform plays a pivotal role in advancing healthcare outcomes by streamlining the research process and accelerating the development of new treatments and therapies. Researchers benefit from increased efficiency, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through NovoClinical contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. The platform's capability to integrate real-world data and electronic health records (EHRs) also facilitates post-marketing surveillance and pharmacovigilance, contributing to drug safety monitoring and regulatory compliance practices.
NovoClinical is an EDC software that allows researchers and trial coordinators to manage clinical trials more efficiently and effectively. By prioritizing patient engagement, ensuring data security, and facilitating collaboration, NovoClinical streamlines the research process and improves healthcare outcomes. With its powerful capabilities and dedication to advancing evidence-based medicine, NovoClinical is a decisive force in the future of clinical research.
57. TriNetX
TriNetX, the leading provider of EDC software, offers a powerful platform that brings efficiency and effectiveness to the research process. With TriNetX, researchers and trial coordinators can seamlessly manage all aspects of a clinical trial, from data collection to study administration, in a centralized and user-friendly environment.
One of the key advantages of TriNetX's EDC software is its ability to enhance patient engagement and compliance. By leveraging innovative tools and features, TriNetX ensures that participants remain actively involved throughout the trial, ultimately leading to more reliable data. Remote patient monitoring, simplified data collection through patient-reported outcomes, and educational materials are just some of the ways TriNetX empowers researchers to foster participant researcher understanding and communication, improving the overall patient experience; TriNetX's platform increases the likelihood of study completion while maintaining the accuracy and reliability of trial results.
Data security and compliance are paramount in the world of clinical trials, and TriNetX recognizes this critical aspect. With robust measures in place, including data encryption, access controls, and comprehensive audit trails, TriNetX's EDC software prioritizes data privacy and meets the stringent requirements of regulatory bodies such as HIPAA and GDPR. This commitment to maintaining the security and confidentiality of patient information instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory authorities.
TriNetX's EDC software is a game-changer in the field of clinical trials, streamlining the research process and accelerating the development of new treatments and therapies. By providing researchers with powerful tools and functionalities, TriNetX empowers them to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, the data collected through TriNetX's platform contributes to evidence-based medicine, allowing healthcare providers to make informed treatment decisions and improve patient outcomes. By integrating real-world data and electronic health records (EHRs), TriNetX enables the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, further enhancing drug safety monitoring and regulatory compliance practices.
TriNetX's EDC software is a solution that brings unparalleled efficiency and effectiveness to the clinical trial process. By prioritizing patient engagement, ensuring data security, and empowering researchers with advanced tools, TriNetX enables the seamless management of trials and the generation of high-quality data. With TriNetX, clinical research looks promising, with faster advancements in healthcare and improved patient outcomes on the horizon.
58. Formedix Onyx
Formedix Onyx is designed to streamline the research process, offering a comprehensive platform that empowers researchers, trial coordinators, and healthcare providers to efficiently collect, manage, and analyze patient data, ensuring the successful completion of every clinical trial.
With a focus on engaging and retaining participants, Formedix Onyx incorporates innovative tools to enhance patient compliance and satisfaction. Through remote patient monitoring and patient-reported outcomes, the software simplifies data collection while providing educational materials to foster participant understanding. By prioritizing patient experience, Onyx creates a seamless working environment that increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and Formedix Onyx is dedicated to protecting sensitive patient information. With robust data encryption, access controls, and comprehensive audit trails, Onyx ensures the privacy and integrity of data, complying with HIPAA and GDPR regulations. This commitment to data security fosters trust among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
Formedix Onyx goes beyond simplifying data management and analysis; it accelerates the development of new treatments, therapies, and medical devices. By facilitating efficient study conduct, Onyx enables faster approvals and the availability of life-saving interventions. Moreover, the data collected through Onyx contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. Integrating real-world data and electronic health records (EHRs), Onyx enhances post-marketing surveillance and pharmacovigilance, ensuring drug safety monitoring and regulatory compliance.
Formedix Onyx sets a new standard for clinical trial management with its advanced EDC software. By streamlining the research process, ensuring data security and compliance, and empowering researchers and healthcare providers, Onyx drives the development of groundbreaking treatments and improves patient outcomes. With its intuitive interface and comprehensive functionalities, Formedix Onyx leads the way to a more efficient and effective future of healthcare.
59. NOVEXAS EDC
Novexas EDC, the innovative electronic data capture (EDC) software, helps clinicians in running clinical trials on well oiled rails. With its cutting-edge features and user-friendly interface, Novexas EDC empowers researchers and trial coordinators to streamline their processes and optimize study outcomes.
At the heart of Novexas EDC lies its ability to simplify data collection and management. The software provides a centralized platform where researchers can efficiently track and analyze patient data, manage study protocols, and automate administrative tasks. By eliminating the need for manual data entry and paperwork, Novexas EDC saves time and resources while minimizing the likelihood of errors. This enhances data accuracy and reliability, enabling researchers to make informed decisions based on real-time access to trial data.
Patient engagement and participation are critical factors in the success of clinical trials. Novexas EDC recognizes this importance and offers tools to enhance patient compliance and satisfaction. Through remote patient monitoring and patient-reported outcomes, the software enables researchers to collect data seamlessly and foster participant understanding. Moreover, Novexas EDC provides educational materials to engage participants and improve their overall experience. By prioritizing patient-centric features, Novexas EDC increases the likelihood of study completion and ensures the generation of reliable data.
Security and compliance are paramount in the world of clinical trials, and Novexas EDC excels in these areas. The software platform prioritizes data privacy and adheres to strict regulatory standards like HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails are implemented to safeguard sensitive patient information. This commitment to data security fosters trusts among stakeholders and facilitates seamless collaboration between researchers, sponsors, and regulatory bodies.
Novexas EDC represents a transformative solution for running clinical trials. By streamlining the research process, optimizing data collection and management, and prioritizing patient engagement, Novexas EDC accelerates the development of new treatments and therapies. The software's functionalities not only improve the efficiency of clinical trials but also contribute to evidence-based medicine, allowing healthcare providers to make informed treatment decisions and ultimately enhance patient outcomes. With Novexas EDC, researchers can navigate the complexities of clinical trials with ease, enabling breakthrough advancements in the medical field.
60. Clinion
Clinion, a revolutionary EDC software, is for streamlining processes for complicated clinical trials. This powerful platform empowers researchers and trial coordinators to efficiently collect and manage data, administer studies, and collaborate seamlessly. Clinion's user-friendly interface and real-time data access create an intuitive environment for researchers to track and analyze patient data effectively.
One of the key challenges in clinical trials is engaging and retaining participants to generate reliable data. Clinion addresses this challenge head-on by offering tools that enhance patient engagement and compliance. With features like remote patient monitoring and simplified data collection through patient-reported outcomes, Clinion ensures that participants feel supported throughout the trial process. By providing educational materials and fostering participant understanding, Clinion focuses on improving the overall patient experience and satisfaction, ultimately increasing the likelihood of study completion and enhancing the accuracy and reliability of trial results.
Data security and compliance are of paramount importance in clinical trials, considering the sensitivity of patient information. Clinion takes data privacy seriously and adheres to HIPAA and GDPR regulations. The platform offers robust data encryption, access controls, and comprehensive audit trails, instilling confidence among stakeholders. This commitment to data security fosters seamless collaboration between researchers, sponsors, and regulatory bodies, allowing for a more efficient and secure research environment.
With its ability to streamline the research process and accelerate the development of new treatments and therapies, Clinion plays a crucial role in advancing medical interventions. By conducting studies more efficiently and obtaining faster approvals, researchers can bring life-saving interventions to patients sooner. Furthermore, the data collected through Clinion contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. Additionally, Clinion facilitates the integration of real-world data and electronic health records (EHRs), allowing for the analysis of larger datasets and promoting drug safety monitoring and regulatory compliance practices.
Clinion's EDC software guides clinical trials towards successful completion, efficiently. By streamlining the research process, improving patient engagement, ensuring data security, and facilitating collaboration, Clinion empowers researchers to conduct studies more efficiently and effectively. The platform's impact extends beyond clinical trials, contributing to evidence-based medicine and the overall advancement of healthcare. With Clinion, clinical research is automated and continues to revolutionize the way trials are conducted and data is collected and analyzed.
61. DSG eCaseLink
DSG eCaseLink emerges as a transformative force, restructuring the way clinical trials are conducted. With its robust and intuitive electronic data capture (EDC) software, DSG eCaseLink empowers researchers and trial coordinators to streamline the entire trial process, enhancing efficiency, accuracy, and patient outcomes.
At the heart of DSG eCaseLink lies its user-friendly platform, designed to simplify data collection, management, and analysis. With seamless integration and real-time data access, researchers can effortlessly track and analyze patient data, ensuring a comprehensive understanding of the trial's progress. This centralized hub fosters collaboration among stakeholders, facilitating efficient study administration and protocol management. By harnessing the power of DSG eCaseLink, researchers can focus their energy on generating reliable data and advancing medical knowledge.
Patient engagement and compliance are vital components of successful clinical trials. DSG eCaseLink understands this, incorporating tools to enhance participant engagement and retention. Through remote patient monitoring and patient-reported outcomes, researchers can capture data efficiently while minimizing the burden on participants. Furthermore, the platform provides educational resources to foster participant understanding, empowering patients to actively contribute to their own healthcare journey. By placing patient experience and satisfaction at the forefront, DSG eCaseLink paves the way for improved study completion rates and more accurate trial results.
Data security is of paramount importance in clinical trials, and DSG eCaseLink prioritizes the privacy and compliance of sensitive patient information. The platform adheres to stringent data security standards, including HIPAA and GDPR, employing robust data encryption, access controls, and comprehensive audit trails. This unwavering commitment to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
DSG eCaseLink's EDC software offers a futuristic solution for running clinical trials. By streamlining the research process, accelerating the development of new treatments, and ensuring faster approvals, this platform drives innovation in the healthcare industry. Moreover, the data collected through DSG eCaseLink contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. With its ability to integrate real-world data and electronic health records (EHRs), DSG eCaseLink supports post-marketing surveillance and pharmacovigilance, fostering drug safety monitoring and regulatory compliance practices.
DSG eCaseLink's EDC software is a trendsetter in the realm of clinical research. By simplifying data capture, facilitating advanced analytics, prioritizing patient engagement, and ensuring data security, this platform empowers researchers to conduct more efficient and effective trials. As DSG eCaseLink pushes the boundaries of clinical research, it paves the way for a future where healthcare decisions are grounded in data-driven insights and personalized treatments, ultimately improving the lives of countless patients around the world.
62. OpenClinica Participate
OpenClinica Participate is an exceptional EDC software that has modernised clinical trials and research. This platform offers a comprehensive suite of tools and features designed to streamline the entire trial process, from data collection to participant management, and study administration. With OpenClinica Participate, researchers and trial coordinators can effortlessly track and analyze patient data, manage study protocols, and automate administrative tasks. The platform's user-friendly interface fosters seamless collaboration and provides real-time access to critical trial information.
One of the key strengths of OpenClinica Participate lies in its commitment to enhancing participant engagement and retention throughout the trial journey. By leveraging innovative tools, this software facilitates remote patient monitoring, simplifies data collection through patient-reported outcomes, and delivers educational materials to ensure participant comprehension. OpenClinica Participate is dedicated to improving the overall patient experience and satisfaction, ultimately increasing the likelihood of study completion and enhancing the accuracy and reliability of trial results.
Security and compliance are paramount in the realm of clinical trials, and OpenClinica Participate understands this imperative. The platform prioritizes data privacy and adheres to stringent regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure the utmost protection of sensitive patient information. This unwavering commitment to data security instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
OpenClinica Participate's impact extends far beyond individual trials. By streamlining the research process and accelerating the development of new treatments, therapies, and medical devices, this software platform contributes to the advancement of healthcare as a whole. The functionalities provided by OpenClinica Participate empower researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Additionally, the data collected through this platform aids in evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. OpenClinica Participate also facilitates the integration of real-world data and electronic health records (EHRs), allowing for the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, thereby enhancing drug safety monitoring and regulatory compliance practices.
OpenClinica Participate is an extraordinary EDC software platform that has transformed the approach to clinical trials. With its streamlined processes, participant engagement features, and robust security measures, OpenClinica Participate empowers researchers and trial coordinators to conduct more efficient and reliable trials with its streamlined processes, participant engagement features, and robust security measures. By leveraging the power of this platform, the research community can accelerate medical advancements and improve patient care on a global scale.
63. REDCap Mobile App
The appearance of Electronic Data Capture (EDC) software has disrupted how clinical trials are conducted. Among the various platforms available, the REDCap Mobile App stands out as a powerful tool contributing to the robustness of the entire trial process.
REDCap Mobile App offers a comprehensive solution for data collection, management, and analysis. Its user-friendly interface allows researchers and trial coordinators to effortlessly capture patient data electronically, eliminating the need for cumbersome paper-based processes. This not only saves time and resources but also enhances data accuracy, reducing the likelihood of errors. By simplifying data collection, REDCap Mobile App enables researchers to focus more on analysis and interpretation, driving scientific discoveries and medical advancements.
One of the key advantages of the REDCap Mobile App lies in its ability to enhance patient engagement and participation in clinical trials. With its mobile accessibility and user-friendly interfaces, patients can easily provide their data and actively participate in research studies. This accessibility promotes inclusivity and diversity in trial populations, leading to more representative and generalizable results. By incorporating patient-reported outcomes, the app empowers patients to play a more active role in their own healthcare, ultimately improving patient satisfaction and treatment outcomes.
Data security and privacy are paramount in clinical trials, and the REDCap Mobile App prioritizes these concerns. The platform adheres to strict data protection regulations and provides robust encryption, access controls, and audit trails. These security measures instill confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies. By ensuring the integrity and confidentiality of patient data, the REDCap Mobile App enables researchers to focus on advancing medical knowledge without compromising privacy.
The REDCap Mobile App comes out as an out-of-the-ordinary tool for managing clinical trials. Its intuitive interface, patient engagement features, and robust data security measures make it an indispensable software for researchers and trial coordinators. By leveraging the power of EDC software, the app accelerates the pace of scientific discovery, facilitates more efficient trial management, and ultimately improves healthcare outcomes for patients worldwide. With the REDCap Mobile App, the future of clinical research is brighter than ever.
64. MACRO eClinical
MACRO eClinical is remoulding the management of clinical trials with its advanced EDC software solutions. These platforms, such as Clinical Studio and Medidata Rave EDC, have transformed the way researchers collect, manage, and analyze data, resulting in improved efficiency, accuracy, and patient engagement.
Clinical Studio, a comprehensive cloud-based platform, serves as a centralized hub for all aspects of clinical trials. Its user-friendly interface enables seamless collaboration and real-time data access. Through tools that enhance patient engagement and compliance, Clinical Studio simplifies data collection, offers remote patient monitoring, and provides educational materials. By prioritizing data privacy and compliance with regulations like HIPAA and GDPR, Clinical Studio instills confidence among stakeholders, facilitating collaboration between researchers, sponsors, and regulatory bodies. This innovative software platform streamlines the research process, expedites approvals, and contributes to evidence-based medicine.
Similarly, Medidata Rave EDC has made significant contributions to clinical trial management and healthcare services. By enabling electronic data capture, Medidata Rave eliminates paper-based processes, saving time, reducing errors, and enhancing data accuracy. With its advanced analytics tools and real-time data access, researchers can monitor trial progress, make informed decisions, and generate comprehensive reports effortlessly. Moreover, Medidata Rave promotes interoperability by seamlessly integrating with other healthcare systems, enabling a holistic view of patient health and improving patient care and outcomes. This platform's user-friendly interfaces and mobile accessibility enhance patient engagement, leading to a more diverse participant pool and more effective treatments.
MACRO eClinical's EDC software solutions are progressive tools for clinical trial management. These platforms, exemplified by Clinical Studio and Medidata Rave EDC, streamline data capture, enhance collaboration, ensure data privacy, and improve patient engagement. By leveraging the capabilities of these innovative software platforms, researchers can conduct more efficient trials, leading to better healthcare outcomes for patients. With their contributions to the research process, these EDC software solutions are at the forefront of transforming clinical trials and shaping the future of evidence-based medicine.
65. Viedoc
Viedoc is the next-generation EDC software that is gradually transforming the clinical trial landscape. Viedoc offers a cutting-edge platform that empowers researchers and trial coordinators to streamline their processes and maximize efficiency. With its intuitive interface and robust features, Viedoc is reshaping the way clinical trials are conducted.
One of the key advantages of Viedoc is its comprehensive data management capabilities. The platform simplifies data collection, storage, and analysis, providing a centralized hub for all trial-related information. Researchers can seamlessly track and monitor patient data, manage study protocols, and automate administrative tasks. This not only saves time and resources but also improves data accuracy and reliability. Viedoc's user-friendly interface promotes collaboration and ensures real-time access to critical trial data.
In addition to its data management prowess, Viedoc prioritizes patient engagement and compliance. The software offers tools and features designed to enhance participant experience and foster long-term involvement in clinical trials. Remote patient monitoring, patient-reported outcomes, and educational materials are seamlessly integrated into the platform, enabling researchers to collect data more efficiently and ensuring participants feel supported throughout the trial journey. By placing patient satisfaction at the forefront, Viedoc increases the likelihood of study completion and produces more reliable trial results.
When it comes to data security and compliance, Viedoc sets the bar high. The platform adheres to stringent privacy regulations, including HIPAA and GDPR, ensuring that sensitive patient information is protected. Robust data encryption, access controls, and comprehensive audit trails bolster the security measures in place. This commitment to data privacy instills confidence among stakeholders and facilitates smooth collaboration between researchers, sponsors, and regulatory bodies. With Viedoc, researchers can focus on advancing medical knowledge and developing life-saving interventions, knowing that their data is secure and their compliance requirements are met.
Viedoc with its comprehensive data management capabilities, focuses on patient engagement, and commitment to data security making it an invaluable tool for researchers and trial coordinators. By leveraging the power of Viedoc, clinical trials can be conducted more efficiently, leading to faster approvals, improved patient outcomes, and ultimately, advancements in medical science. Viedoc is positively setting a new standard for EDC software in the industry.
66. StudyManager
StudyManager for clinical trials is empowering researchers and trial coordinators with an innovative Electronic Data Capture (EDC) software platform. This cloud-based solution brings together all the essential components of a clinical trial, offering a centralized hub for data management, patient engagement, and study administration. With StudyManager, conducting clinical trials has never been more efficient, accurate, and secure.
EDC software lies at the heart of StudyManager's success. By digitizing the data collection process, researchers can bid farewell to time-consuming and error-prone paper-based methods. StudyManager's intuitive interface allows seamless data entry and real-time access, ensuring data accuracy and reducing the likelihood of errors. The software's advanced functionalities enable researchers to streamline data management, collaborate effectively, and make informed decisions based on up-to-date information.
Patient engagement is a key driver of successful clinical trials, and StudyManager excels in this aspect. The platform incorporates tools specifically designed to enhance participant involvement and compliance. Through remote patient monitoring and patient-reported outcomes, StudyManager simplifies data collection while ensuring participants remain engaged throughout the trial. The software also provides educational materials to foster participant understanding, leading to improved patient experience and satisfaction.
Security and compliance are of utmost importance in clinical trials, and StudyManager recognizes this. The platform adheres to stringent data privacy regulations, including HIPAA and GDPR, guaranteeing the confidentiality and integrity of patient information. StudyManager's robust data encryption, access controls, and comprehensive audit trails instill confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
StudyManager's impact extends beyond individual trials. By accelerating the research process and facilitating faster approvals, this software platform accelerates the development of new treatments, therapies, and medical devices. The data collected through StudyManager contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and improve patient outcomes. Furthermore, StudyManager's seamless integration with other healthcare systems, such as electronic health records (EHRs), allows for comprehensive data analysis and real-time insights, leading to improved patient care and post-marketing surveillance.
StudyManager's EDC software provides researchers and trial coordinators with a comprehensive, secure, and efficient platform, StudyManager enhances data collection, analysis, and management processes. With its focus on patient engagement, data security, and seamless integration, StudyManager is propelling the field of clinical trials forward, bringing us closer to safer, more effective healthcare solutions for all.
67. Target e*Data
Target e*Data offers an innovative EDC software solution that renovates the way data is collected, analyzed, and managed. With its comprehensive suite of features, Target e*Data simplifies the clinical trial process, enabling researchers and trial coordinators to streamline their operations and drive accurate results.
Target e*Data's EDC software serves as a centralized hub for all trial-related activities, providing a seamless platform for data collection, patient management, and study administration. This user-friendly interface ensures efficient tracking and analysis of patient data, allowing researchers to gain valuable insights in real-time. By automating administrative tasks and study protocols, the software empowers researchers to focus on what truly matters: advancing medical knowledge and improving patient outcomes.
One of the key advantages of Target e*Data's EDC software lies in its ability to enhance patient engagement and compliance. By incorporating tools for remote patient monitoring, patient-reported outcomes, and educational materials, the software creates an environment that fosters participant understanding and commitment. This not only improves the overall patient experience but also increases the likelihood of study completion, generating reliable and impactful data.
Data security and compliance are of utmost importance in clinical trials, and Target e*Data understands this critical aspect. The software platform prioritizes data privacy and meets stringent regulatory standards, ensuring that sensitive patient information remains secure. With robust data encryption, access controls, and comprehensive audit trails, stakeholders can collaborate with confidence, knowing that their data is protected and handled with the utmost care.
Target e*Data's EDC software empowers researchers and trial coordinators to conduct clinical trials with greater efficiency, accuracy, and participant engagement. By providing a comprehensive solution for data collection and management, the software enables researchers to unlock valuable insights and drive medical advancements. With its commitment to data security and compliance, Target e*Data sets a new standard in the field of clinical trials, facilitating collaboration and trust among stakeholders.
68. PHT LogPad
The PHT LogPad, is an innovative EDC software for revamping the process of running clinical trials. This innovative platform reimagines the way data is collected, managed, and analyzed, offering a seamless and efficient solution for researchers and trial coordinators alike.
The PHT LogPad simplifies the complex task of data collection through its user-friendly interface. Gone are the days of cumbersome paper-based processes, as this software allows for electronic data capture, eliminating errors and enhancing accuracy. With real-time data access and advanced analytics tools, researchers can monitor study progress and make informed decisions with ease. The platform's centralized hub ensures that patient information is securely stored and readily available for analysis, promoting collaboration and speeding up the research process.
One of the key strengths of the PHT LogPad lies in its focus on patient engagement. By providing a mobile-accessible platform with multilingual support, it encourages diverse participation and enhances the generalizability of trial results. The LogPad also incorporates educational materials, empowering participants and fostering a deeper understanding of the study. Through patient-reported outcomes and remote monitoring capabilities, the platform enables researchers to collect data efficiently and comprehensively, ultimately improving the patient experience and satisfaction.
Data security and compliance are paramount in clinical trials, and the PHT LogPad prioritizes these aspects. With robust data encryption, access controls, and comprehensive audit trails, the platform ensures the confidentiality and integrity of sensitive patient information. This commitment to privacy instills confidence among stakeholders, facilitating seamless collaboration between researchers, sponsors, and regulatory bodies.
The PHT LogPad EDC software represents a significant advancement in the field of clinical trials. By streamlining data capture, enhancing patient engagement, and prioritizing data security, it empowers researchers to conduct studies more efficiently and generate reliable results. With its user-friendly interface and advanced analytics capabilities, the LogPad has the potential to accelerate the development of life-saving interventions and improve patient outcomes. Embrace the future of clinical trials with the PHT LogPad and unlock new possibilities in medical research.
69. MACRO Mobile
Clinical trial demands efficiency, accuracy, and patient engagement for its successful execution. And EDC software is an indispensible pivot in streamlining research processes. Amongst so many innovative options for EDC software systems, MACRO Mobile is a groundbreaking platform that is transforming the way clinical trials are conducted, bringing remarkable advancements to the forefront of the industry.
At the core of MACRO Mobile lies its comprehensive EDC software, which serves as the backbone for data collection, management, and analysis. This user-friendly platform empowers researchers and trial coordinators with a centralized hub that seamlessly tracks patient data, manages study protocols, and automates administrative tasks. By leveraging the intuitive interface and real-time data access, collaboration between stakeholders is enhanced, leading to accelerated trial timelines and improved overall efficiency.
One of the key strengths of MACRO Mobile is its unwavering commitment to patient engagement and compliance. The platform incorporates innovative tools that promote participant understanding, remote patient monitoring, and simplified data collection through patient-reported outcomes. Additionally, educational materials are provided to foster patient involvement, ensuring a smooth and positive experience. By placing patient satisfaction at the forefront, MACRO Mobile not only increases the likelihood of study completion but also enhances the accuracy and reliability of trial results.
Data security and compliance are of utmost importance in clinical trials, and MACRO Mobile rises to the occasion. Built with robust measures to safeguard sensitive patient information, the platform adheres to stringent privacy regulations such as HIPAA and GDPR. Through comprehensive data encryption, access controls, and detailed audit trails, MACRO Mobile instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
MACRO Mobile's innovative EDC software provides a way to upgrade legacy management systems for clinical trials. By streamlining research processes, expediting approvals, and improving patient outcomes. Furthermore, MACRO Mobile's integration capabilities enable the analysis of larger datasets, contributing to post-marketing surveillance and drug safety monitoring. With its unwavering commitment to efficiency, accuracy, and patient engagement, MACRO Mobile sets a new standard in the realm of clinical trial management.
70. Clincase Mobile
Clincase Mobile leverages the power of mobile technology, to empower researchers and trial coordinators to conduct studies more efficiently and effectively, advancing medical research and improving patient outcomes.
With Clincase Mobile, data collection becomes seamless and convenient. Researchers can easily capture and record patient information directly on their mobile devices, eliminating the need for cumbersome paper-based processes. This not only saves time and resources but also enhances data accuracy, reducing the likelihood of errors. The mobile interface provides an intuitive and user-friendly experience, allowing researchers to efficiently track and manage patient data on the go.
One of the standout features of Clincase Mobile is its ability to enhance patient engagement and participation. The software offers innovative tools for remote patient monitoring, patient-reported outcomes, and educational materials. By providing patients with convenient ways to participate in research studies, Clincase Mobile fosters a sense of empowerment and involvement. This not only improves the overall patient experience but also ensures a more diverse participant pool, leading to more representative and reliable trial results.
Data security and compliance are of utmost importance in clinical trials, and Clincase Mobile prioritizes the protection of sensitive patient information. The software platform incorporates robust data encryption, access controls, and comprehensive audit trails to safeguard data privacy. By complying with industry regulations and standards, such as HIPAA and GDPR, Clincase Mobile instills confidence among stakeholders and enables seamless collaboration between researchers, sponsors, and regulatory bodies.
Clincase Mobile with its mobile capabilities streamlines data collection, enhances patient engagement, and ensures data security, accelerating medical research and improving patient outcomes. Its EDC software, empowers researchers to conduct studies more efficiently and effectively, paving the way for groundbreaking advancements in healthcare.
71. Medidata Coder
Medidata Coder is a cutting-edge EDC software that helps clinical trial organizations to upgrade their management processes, bringing remarkable advancements to the field of medical research. By providing a robust and intuitive platform for electronic data capture (EDC), Medidata Coder simplifies data collection, analysis, and management, empowering researchers and medical professionals to make significant strides in healthcare innovation.
At the core of Medidata Coder is its ability to streamline the clinical trial process by digitizing data capture. Researchers can effortlessly collect and record patient information electronically, eliminating the need for traditional paper-based methods. This transition not only saves valuable time and resources but also minimizes the risk of errors and enhances the accuracy of data. With Medidata Coder, researchers can efficiently manage data and foster collaboration among research teams, centralizing data in a secure and standardized format.
One of the standout features of Medidata Coder is its advanced analytics and reporting capabilities. Researchers have real-time access to trial data, enabling them to closely monitor the progress of their studies and make informed decisions promptly. The platform's built-in analytics tools empower researchers to perform complex data analyses, identify trends, and effortlessly generate comprehensive reports. This analytical prowess leads to quicker data-driven insights and enhances the overall efficiency of clinical trials.
Medidata Coder also places a strong emphasis on patient engagement and participation. The platform offers user-friendly interfaces, mobile accessibility, and multilingual support, making it easier for patients to provide their data and actively participate in research studies. By enhancing patient accessibility and inclusivity, Medidata Coder enables a more diverse participant pool, leading to improved generalizability of trial results and ultimately fostering better-informed healthcare decisions and more effective treatments.
Medidata Coder is an exceptional EDC software that revolutionizes clinical trial management and drives healthcare innovation. By digitizing data capture, enabling advanced analytics, and prioritizing patient engagement, Medidata Coder empowers researchers to conduct more efficient and effective trials, leading to improved healthcare outcomes for patients. With its comprehensive suite of features, Medidata Coder is propelling medical research into the future and transforming the way we approach clinical trials.
72. ArisGlobal agCDISC
ArisGlobal agCDISC, is a pioneering solution that figures among the top EDC software solutions for clinical trials. Its Electronic Data Capture (EDC) software with its robust capabilities and user-friendly interface, empowers researchers and trial coordinators to streamline data collection, enhance patient engagement, and ensure data integrity throughout the trial process.
At the core of agCDISC lies its comprehensive EDC software, which simplifies the collection and management of patient data. By replacing traditional paper-based processes, the software eliminates the tedious manual work and potential errors associated with data entry. Researchers can now capture and record patient information electronically, enabling real-time access and analysis. This not only saves valuable time and resources but also enhances the accuracy and reliability of trial data.
agCDISC goes beyond data capture and offers tools to foster participant engagement and compliance. The software enables remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational resources for enhanced participant understanding. By prioritizing the patient experience, agCDISC ensures greater participant retention and higher-quality data, ultimately bolstering the success of clinical trials.
Data security and compliance are paramount concerns in clinical trials, and agCDISC addresses them with unwavering commitment. The software adheres to industry regulations such as HIPAA and GDPR, and employs robust data encryption, access controls, and comprehensive audit trails. These security measures instill confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies while maintaining the utmost confidentiality of patient data.
agCDISC's impact extends far beyond individual trials. By accelerating the development of new treatments, therapies, and medical devices, the software contributes to the advancement of healthcare on a larger scale. Researchers can conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Moreover, the data collected through agCDISC facilitates evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes.
ArisGlobal agCDISC's EDC software offers a game-changing software that streamlines data capture, enhances patient engagement, and does not compromise data security. agCDISC empowers researchers and trial coordinators to conduct trials more efficiently and effectively. By leveraging the power of agCDISC, the healthcare industry can usher in a new era of innovation and improved patient care.
73. Clinical Conductor
Clinical Conductor is an innovative EDC software that remodels the management of clinical trials. By providing a robust and intuitive platform, Clinical Conductor facilitates researchers and trial coordinators to effectively manage every aspect of their studies. With its comprehensive suite of features, this software simplifies data collection, streamlines patient management, and automates study administration.
One of the key advantages of Clinical Conductor is its focus on enhancing patient engagement and compliance. Recognizing that successful trial completion hinges on participant involvement, Clinical Conductor integrates tools that facilitate remote patient monitoring and simplify data collection through patient-reported outcomes. Moreover, it offers educational materials to foster participant understanding and improve the overall patient experience. By prioritizing patient engagement, Clinical Conductor ensures that researchers gather reliable and accurate data, increasing the likelihood of study completion and generating meaningful results.
Security and compliance are paramount in the realm of clinical trials, and Clinical Conductor recognizes this imperative. With stringent data privacy measures, including robust encryption, access controls, and comprehensive audit trails, this software platform prioritizes data protection. By adhering to industry regulations such as HIPAA and GDPR, Clinical Conductor instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
In addition to its efficacy in managing clinical trials, Clinical Conductor contributes to the development of life-saving interventions and evidence-based medicine. By streamlining the research process, this software platform enables researchers to conduct studies more efficiently, resulting in faster approvals and the availability of vital treatments, therapies, and medical devices. Furthermore, the data collected through Clinical Conductor facilitates post-marketing surveillance and pharmacovigilance, enabling the integration of real-world data and electronic health records (EHRs). This integration promotes the analysis of larger datasets, contributing to drug safety monitoring and regulatory compliance practices.
In essence, Clinical Conductor has advanced functionalities, user-friendly interface, and unwavering commitment to data security and patient engagement. This EDC software propels research forward, accelerates treatment development, and improves patient outcomes. Researchers and trial coordinators can leverage the power of Clinical Conductor to conduct more efficient trials, ultimately advancing the frontiers of healthcare.
74. Medidata Balance
Medidata Balance EDC software provides researchers and trial coordinators with a powerful platform for managing and analyzing patient data. This comprehensive cloud-based solution offers a centralized hub for data collection, patient management, and study administration, simplifying the entire clinical trial process.
With Medidata Balance, engaging and retaining participants in clinical trials becomes more manageable. The software incorporates tools to enhance patient engagement and compliance, such as remote patient monitoring and patient-reported outcomes. By fostering participant understanding through educational materials, Medidata Balance ensures a smooth and positive patient experience by fostering participant understanding through educational materials. This dedication to patient satisfaction increases the likelihood of study completion and enhances the accuracy and reliability of trial results.
Data security and compliance are of paramount importance in clinical trials, and Medidata Balance prioritizes these aspects. The platform adheres to strict data privacy regulations, including HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails provide a secure environment for sensitive patient data. This commitment to data security fosters confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
Medidata Balance EDC software not only streamlines the research process but also accelerates the development of new treatments, therapies, and medical devices. Its functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Additionally, the data collected through Medidata Balance contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. The platform also facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, thus enhancing drug safety monitoring and regulatory compliance practices.
Medidata Balance EDC software has transformed clinical trial management, providing researchers with a comprehensive and efficient platform for data collection, analysis, and management platform. By prioritizing patient engagement, ensuring data security and compliance, and promoting evidence-based medicine, Medidata Balance empowers researchers to conduct successful trials and improve healthcare outcomes for patients.
75. DrugDev EDC
DrugDev EDC software has powerful capabilities and user-friendly interface. This comprehensive platform simplifies the complex process of running clinical trials, empowering researchers and coordinators to track and analyze patient data, manage study protocols, and automate administrative tasks. With DrugDev EDC software, the entire trial journey becomes seamless and efficient.
One of the key strengths of DrugDev EDC software is its focus on patient engagement and compliance. The platform offers a range of tools to enhance participant involvement, including remote patient monitoring and simplified data collection through patient-reported outcomes. Furthermore, educational materials are provided to foster participant understanding, ensuring a smooth and informative experience. By prioritizing patient experience and satisfaction, DrugDev EDC software not only increases the likelihood of study completion but also improves the accuracy and reliability of trial results.
Data security and compliance are paramount in clinical trials, and DrugDev EDC software is designed with this in mind. The platform prioritizes data privacy, adhering to strict regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails are implemented to ensure the highest level of security. This commitment to protecting sensitive patient data instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
DrugDev EDC software is a tool for running clinical trials and a catalyst for the development of new treatments, therapies, and medical devices. By streamlining the research process and enabling more efficient studies, this software platform accelerates the approvals and availability of life-saving interventions by streamlining the research process and enabling more efficient studies by streamlining the research process and enabling more efficient studies. Additionally, the data collected through DrugDev EDC software contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. The platform also facilitates the integration of real-world data and electronic health records, further enhancing drug safety monitoring and regulatory compliance practices.
DrugDev EDC software emphasises on patient engagement, and its robust security measures make it an indispensable tool for researchers and trial coordinators. By harnessing the power of DrugDev EDC software, the clinical trial process becomes more efficient, reliable, and impactful, ultimately benefiting both researchers and patients alike.
76. Clinical Studio
Clinical Studio, is an innovative cloud-based platform, and has comprehensive suite of features that streamlines processes. At the core of Clinical Studio is its cutting-edge electronic data capture (EDC) software, designed to simplify data collection, patient management, and study administration. This intuitive platform empowers researchers and trial coordinators to track and analyze patient data, manage study protocols, and automate administrative tasks seamlessly.
One of the key strengths of Clinical Studio lies in its commitment to enhancing patient engagement and compliance. By leveraging tools such as remote patient monitoring and patient-reported outcomes, the platform facilitates data collection while ensuring participant understanding and satisfaction. The software's emphasis on the smooth functioning of trial components increases the likelihood of study completion and enhances the accuracy and reliability of trial results.
In the times where data privacy and compliance are paramount, Clinical Studio sets the bar high. The platform places a strong emphasis on data security, complying with the stringent regulations set forth by HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails provide stakeholders with the confidence they need to collaborate seamlessly. This trust and collaboration between researchers, sponsors, and regulatory bodies contribute to the success of clinical trials.
By streamlining the research process and expediting the development of new treatments and therapies, Clinical Studio accelerates medical advancements. Its functionalities enable researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Furthermore, the data collected through Clinical Studio supports evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. The platform also facilitates the integration of real-world data and electronic health records (EHRs), enabling the analysis of larger datasets for post-marketing surveillance and pharmacovigilance, contributing to drug safety monitoring and regulatory compliance practices.
Clinical Studio's comprehensive platform and its state-of-the-art EDC software have brought a new level of efficiency to the clinical trial landscape. It not only simplifies the data capture and management processes but also promotes collaboration, data security, and compliance. With its powerful capabilities, Clinical Studio empowers researchers and stakeholders to make significant strides in medical research and healthcare delivery, ultimately improving the lives of patients worldwide.
77. Advarra EDC
Advarra EDC software offers a robust and comprehensive platform that empowers researchers to streamline the entire trial process. With Advarra EDC, the arduous task of data collection, management, and analysis becomes a seamless experience. This cloud-based solution provides a centralized hub for researchers and trial coordinators to efficiently track patient data, manage study protocols, and automate administrative tasks, all while ensuring the highest standards of data privacy and compliance.
The key strength of Advarra EDC software lies in its focus on engaging and retaining participants in clinical trials. Recognizing that generating reliable data hinges upon participant involvement, Advarra EDC integrates tools for enhancing patient engagement and compliance. It enables remote patient monitoring, simplifies data collection through patient-reported outcomes, and provides educational materials to foster participant understanding. By prioritizing patient experience and satisfaction, Advarra EDC creates an environment that increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Data security is of utmost importance in clinical trials, and Advarra EDC software addresses this concern with unwavering commitment. The platform prioritizes data privacy and compliance with regulations such as HIPAA and GDPR. Robust data encryption, access controls, and comprehensive audit trails ensure the integrity and security of sensitive patient information. This dedication to data security instills confidence among stakeholders, enabling seamless collaboration between researchers, sponsors, and regulatory bodies.
With Advarra EDC software, the successful completion of all aspects of a clinical trial becomes a reality. This innovative platform streamlines the research process, accelerates the development of new treatments and therapies, and facilitates evidence-based medicine. Researchers can conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Additionally, the data collected through Advarra EDC contributes to post-marketing surveillance and pharmacovigilance, supporting drug safety monitoring and regulatory compliance practices.
Advarra EDC software offers a comprehensive and secure platform that enhances participant engagement, ensures data privacy platform facilitates data collection by and accelerates medical advancements. Its intuitive interface, powerful features, and stringent data security puts it in a class beyond compare.
78. Castor EDC Mobile
Castor EDC Mobile introduces a powerful and intuitive EDC software designed for mobile devices. This innovative platform empowers researchers and trial coordinators by providing a seamless and efficient solution for data collection, patient management, and study administration. With Castor EDC Mobile, the entire clinical trial process becomes streamlined and accessible at your fingertips.
The user-friendly interface of Castor EDC Mobile ensures that researchers can effortlessly capture and record patient data electronically, eliminating the cumbersome paperwork of traditional methods. By embracing the convenience of mobile devices, Castor EDC Mobile enhances data accuracy, reduces errors, and accelerates the pace of data collection. Researchers can now collect, manage, and analyze data in real-time, allowing for prompt decision-making and the ability to adapt studies based on emerging insights.
Amongst key advantages of Castor EDC Mobile lies its commitment to patient engagement. The platform incorporates features that promote patient compliance, facilitate remote patient monitoring, and provide educational materials to enhance participant understanding. By ensuring a positive and interactive experience for participants, Castor EDC Mobile fosters higher retention rates, generating reliable and robust data for clinical trials.
In addition to its user-centric design, Castor EDC Mobile prioritizes data security and compliance. The platform implements stringent measures to safeguard sensitive patient data, including robust encryption, access controls, and comprehensive audit trails. With Castor EDC Mobile, researchers, sponsors, and regulatory bodies can collaborate seamlessly, confident in the protection of data privacy and the adherence to industry regulations.
Castor EDC Mobile represents awesome advancement in the field of clinical trials. By harnessing the power of mobile devices and combining it with a comprehensive EDC software solution, researchers can unlock new levels of efficiency, accuracy, and patient engagement. This transformative platform empowers researchers to conduct studies with greater ease and confidence, leading to faster approvals, improved treatments, and ultimately, enhanced patient outcomes. With Castor EDC Mobile, the future of clinical trials is at your fingertips.
79. Nextrials Prancer
Nextrials Prancer, the cutting-edge EDC software for clinical trials, has been designed with meticulous attention to detail and a relentless pursuit of efficiency. Prancer offers a comprehensive solution for researchers and trial coordinators, empowering them to navigate the complexities of clinical trials with ease and precision.
At the heart of Prancer lies its ability to streamline the entire trial process, from data collection to patient management and study administration. With its intuitive interface and centralized hub, Prancer provides a seamless experience for tracking and analyzing patient data, managing study protocols, and automating administrative tasks. This not only saves valuable time and resources but also enhances accuracy and reliability throughout the trial journey.
When it comes to patient engagement, Prancer excels with its arsenal of tools designed to foster participation and drive reliable data generation. Leveraging remote patient monitoring, simplified data collection through patient-reported outcomes, and educational materials, Prancer ensures that participants remain actively involved and informed throughout the trial. By prioritizing patient experience and satisfaction, Prancer sets the stage for successful study completion and empowers researchers to unlock invaluable insights.
Without the assurance of data security and compliance no medtech software can gain ground. Prancer stands tall in embodying a steadfast commitment to safeguarding sensitive patient information . With robust data encryption, comprehensive access controls, and meticulous audit trails, Prancer ensures the privacy of patient data while adhering to regulatory requirements such as HIPAA and GDPR. This instills confidence among stakeholders, fostering seamless collaboration between researchers, sponsors, and regulatory bodies.
The advent of Nextrials Prancer represents a significant milestone in the evolution of clinical trials. By streamlining research processes and expediting the development of new treatments and therapies, Prancer paves the way for faster approvals and the availability of life-saving interventions. Moreover, the data collected through Prancer contributes to evidence-based medicine, enabling healthcare providers to make informed treatment decisions and ultimately improve patient outcomes.
With its remarkable capabilities and unwavering dedication to excellence, Nextrials Prancer is poised to reshape the landscape of clinical trials. By harnessing the power of advanced EDC software, researchers can embark on a journey of enhanced efficiency, accuracy, and patient-centricity, ushering in a new era of medical progress and transforming the lives of countless individuals around the globe.
80. RealTime-CTMS
RealTime-CTMS, the innovative software platform for running clinical trials, has effectively disrupted medical research and study management. With its comprehensive suite of features, RealTime-CTMS simplifies and streamlines the entire process, offering researchers and trial coordinators an intuitive and efficient tool to optimize their workflow and achieve successful study outcomes.
One of the standout features of RealTime-CTMS is its advanced electronic data capture (EDC) software, which enables researchers to seamlessly collect and record patient information. By eliminating the need for cumbersome paper-based processes, RealTime-CTMS not only saves valuable time and resources but also enhances data accuracy by reducing the likelihood of errors. This agile platform facilitates real-time data access and collaboration, empowering researchers to track and analyze patient data efficiently while ensuring compliance with data privacy regulations.
RealTime-CTMS goes beyond data capture and offers tools for enhancing patient engagement and compliance throughout the clinical trial journey. With features like remote patient monitoring and patient-reported outcomes, the platform simplifies data collection and fosters participant understanding. By providing educational materials and prioritizing patient satisfaction, RealTime-CTMS creates a supportive environment that increases the likelihood of study completion and improves the accuracy and reliability of trial results.
Furthermore, RealTime-CTMS places paramount importance on data security and compliance. With robust data encryption, access controls, and comprehensive audit trails, the platform ensures the protection of sensitive patient data and complies with strict regulations such as HIPAA and GDPR. This commitment to data security instills confidence among stakeholders and facilitates seamless collaboration between researchers, sponsors, and regulatory bodies.
RealTime-CTMS has successfully come up with software systems for accelerating the development of new treatments, therapies, and medical devices. By streamlining the research process and offering advanced functionalities for data analysis and reporting, the platform enables researchers to conduct studies more efficiently, leading to faster approvals and the availability of life-saving interventions. Additionally, the data collected through RealTime-CTMS contributes to evidence-based medicine, empowering healthcare providers to make informed treatment decisions and improve patient outcomes. With its integration capabilities and emphasis on patient engagement, RealTime-CTMS is paving the way for a future of more effective and patient-centered clinical trials.
Conclusion
To conclude, EDC software platform companies are crucial in the digital transformation era, innovating and shaping industries in remarkable ways. Their platforms streamline data collection, improve data quality, and enhance operational efficiency. They facilitate regulatory compliance, mitigate risk, and accelerate clinical trials, underpinning advancements in the healthcare sector.
Furthermore, EDC software companies aren't merely technology providers, but partners driving business growth, and enabling organizations to navigate complex technological landscapes. While challenges persist, like ensuring data security and managing scalability, their continued evolution and adaptability make them indispensable. Their growth trajectory affirms the significant role they play in shaping a future where data integrity, accessibility, and usability are paramount. As we move forward, these companies' contributions will become even more significant in the grand scheme of digital transformation and data-driven decision making.